- Insulin glulisine
drugbox
IUPAC_name =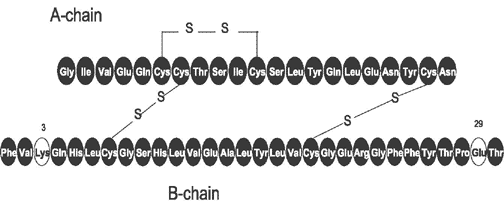
CAS_number = 207748-29-6
ATC_prefix = A10
ATC_suffix = AB06
PubChem =
DrugBank =
C=258|H=384|N=64|O=78|S=6
molecular_weight = 5823
bioavailability =
protein_bound =
metabolism =
elimination_half-life =
excretion =
pregnancy_AU =
pregnancy_US = C
pregnancy_category=
legal_AU =
legal_CA =
legal_UK = POM
legal_US = Rx-only
legal_status =
routes_of_administration = SubcutaneousInsulin glulisine is a rapid-acting
insulin analogue that differs from humaninsulin in that the amino acidasparagine at position B3 is replaced bylysine and the lysine in position B29 is replaced byglutamic acid . Chemically, it is 3B-lysine-29B-glutamic acid-human insulin, has the empirical formula C258H384N64O78S6 and a molecular weight of 5823. It was developed bySanofi-Aventis and sold under the trade name Apidra. When injected subcutaneously, it appears in the blood earlier and at higher concentrations that human insulin. When used as a meal time insulin, the dose should be given within 15 minutes before a meal or within 20 minutes after starting a meal.External links
* [http://products.sanofi-aventis.us/apidra/apidra.html Apidra Homepage]
*http://www.genome.jp/dbget-bin/www_bget?dr:D04540
Wikimedia Foundation. 2010.
