- Insulin aspart
-
Insulin Aspart 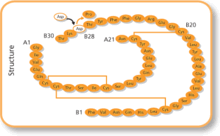
Clinical data AHFS/Drugs.com monograph MedlinePlus a605013 Pregnancy cat. C(US) Legal status ℞-only (US) Routes Subcutaneous Identifiers CAS number 116094-23-6 
ATC code A10AB05 PubChem CID 16132418 DrugBank DB01306 UNII D933668QVX 
KEGG D04475 
ChEMBL CHEMBL1201496 
Chemical data Formula C256H381N65O79S6 Mol. mass 5825.8 g/mol  (what is this?) (verify)
(what is this?) (verify)A fast acting insulin analog (marketed by Novo Nordisk as "NovoLog/NovoRapid") is a man-made form of human insulin. A single amino acid has been slightly changed in its molecular structure. This change helps the fast-acting insulin analog absorb quickly into the bloodstream. As a result, it starts working in minutes, which allows you to take insulin and eat right away. Fast-acting insulin analogs are considered to act similar to the way insulin is released in people without diabetes mellitus.
Novolog allows for a flexible dosing schedule, which allows the patient to adjust their insulin according to any changes in their eating habits.[1]
It was created through recombinant DNA technology so that the amino acid, B28, which is normally proline, is substituted with an aspartic acid residue. This analogue has increased charge repulsion, which prevents the formation of hexamers, to create a faster acting insulin. The sequence was inserted into the yeast genome, and the yeast expressed the insulin analogue, which was then harvested from a bioreactor.
According to JDRF, insulin aspart was approved for marketing in the United States by the Food and Drug Administration in June 2000.[citation needed]
Contents
Chemical properties
The components of insulin aspart are as follows:
- Metal ion – zinc (19.6 ug/mL)
- Buffer – disodium hydrogen phosphate dihydrate (1.25 mg/mL)
- Preservative – m-cresol (1.72 mg/mL) and phenol (1.50 mg/mL)
- Isotonicity agent – glycerin (16 mg/mL) and sodium chloride (0.58 mg/mL).
The pH of insulin aspart is 7.2-7.6.[2]
Action time
The onset of action is approximately 15 minutes, the peak action is reached in 45–90 minutes, and the duration is 3–5 hours. However, as with all insulin, these numbers are based on averages, and vary between individuals due to blood flow, injection site, temperature and exercise.[2][3]
Temperature
It has been debated whether or not insulin aspart (or NovoLog/NovoRapid) should be refrigerated. Recent studies have shown that there is no difference, however insulin should not be stored in intense heat for a long period of time. This causes the hormone to reach a pre- boiling state, and separate. This can change the potency of the insulin, and it would no longer be as effective.
Novolog (vial) should be stored in the refrigerator between 2°C and 8°C (36°F and 46°F), until it is first used. [1] Novolog should never be used if it has been frozen.[4] NovoLog® FlexPen®, vials and cartridges that are being used can be kept at room temperature—below 30°C (86°F)—for up to 28 days. Novolog should not be stored in areas of extreme moisture or where there may be extreme temperatures, like a freezer or car.[1]
Usage
Insulin aspart can be used in CSII pumps and Flexpen, Novopen delivery devices for subcutaneous injection. Additionally, it can be used with an injection port such as the I-port.[5]
Novolog® is used for the treatment of patients with diabetes mellitus to control hyperglycemia.[6] Novolog® has a more rapid onset, and a shorter duration of activity than normal human insulin. Therefore, Novolog® given by injection should normally be used in a regimen with long-acting or intermediate insulin.[6] Novolog® can also be used with external insulin pumps. The insulin should be changed in the reservoir of the insulin pump at least every 6 days, and the infusion sets should be changed at least every 3 days. The following insulin pumps have been used in Novolog® clinical or in vitro studies conducted by Novo Nordisk: Medtronic Paradigm® 512 and 712, Minimed 508, Disetronic® D-TRON® and H-TRON®.[4]
Variations on Insulin Aspart
NovoLog Mix 70/30 is a product marketed by Novo Nordisk which contains 30% insulin aspart and 70% insulin aspart protamine. The insulin aspart protamine portion is a crystalline form of insulin aspart, which delays the action of the insulin, giving it a prolonged absorption profile after injection. The combination of the fast-acting form and the long-acting form allows the patient to receive fewer injections over the course of the day.[7]
The components of NovoLog Mix 70/30 are as follows:
- Metal ion – zinc (19.6 ug/mL)
- Buffer – dibasic sodium phosphate (1.25 mg/mL)
- Preservative – m-cresol (1.72 mg/mL) and phenol (1.50 mg/mL)
- Isotonicity agent – sodium chloride (0.58 mg/mL) and mannitol (36.4 mg/mL)
- Modifying protein – protamine (0.33 mg/mL)
The pH is 7.2-7.44.[2]
NovoLog Mix is marketed to be used with the Novo Nordisk FlexPen.[8] The onset of action is less than 30 minutes, the peak action is reached in 1–4 hours, and the duration is less than 24 hours.[2]
NovoLog® is available in different forms. There are 10-mL vials, 3-mL PenFill® cartridges, and 3mL NovoLog® FlexPens®. The NovoLog® FlexPen® is a way to take NovoLog® via a prefilled, disposable insulin pen delivery system. The NovoLog® FlexPen® can be used for up to 28 days without being refrigerated once it has been used. The large, clear dosing window allows for accurate dose setting and adjustment. NovoLog® is also supplied in 3-mL PenFill® cartridges and can be used in the NovoPen® 3 insulin delivery system.[1]
Side effects
The safety of Novolog in patients with diabetes has been evaluated in several clinical studies. Novolog was compared with regular human insulin, and there was no difference in the frequency of adverse effects between the two treatments. The side effects that are commonly associated with insulin therapy include: allergic reactions, injection site irritation, rashes, and hypoglycemia.[1] The most common side effect is hypoglycemia. Long-term use of insulin, including Novolog®, can cause lipodystrophy at the site of repeated injections or infusion. To reduce the risk of lipodystrophy, rotate the injection sites within the same region. Weight gain can also occur with the use of Novolog® and it has been attributed to anabolic effects of insulin and a decrease in glucosuria. Use of Novolog® has also been associated with sodium retention and edema.[4]
External links
References
- ^ a b c d e "Novolog: most FAQ". http://www.novolog.com/questions-default.asp?s=faqs-6. Retrieved 2011.
- ^ a b c d Crommelin DJA, Sindelar RD, Meibohm B. 2008. Pharmaceutical Biotechnology: Fundamentals and Applications. New York, NY: Informa Healthcare USA, Inc. p 270.
- ^ FDA. NovoLog Insulin Aspart (rDNA origin) Injection. 7 June 2000. http://www.fda.gov/CDER/foi/label/2000/20986lbl.pdf
- ^ a b c "Novolog: insulin aspart (rDNA origin) injection". http://www.novo-pi.com/novolog.pdf. Retrieved 2010-10.
- ^ "Aspart insulin (rDNA origin) injection". Archived from the original on 2007-06-10. http://web.archive.org/web/20070610074313/http://www.nlm.nih.gov/medlineplus/druginfo/medmaster/a605013.html. Retrieved 2007-06-08.
- ^ a b "Novolog: insulin aspart". http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4254b_13_04_KP%20InsulinAspartFDAlabel102005.pdf.
- ^ Rx List: NovoLog Mix 70/30. 6 August 2008. http://www.rxlist.com/novolog-mix-70-30-drug.htm
- ^ Novo Nordisk: NovoLog Mix 70/30. 2008. http://www.novologmix70-30.com/starting-with-novolog-mix.asp
Oral anti-diabetic drugs and Insulin analogs (A10) Insulin K+ ATPMeglitinides/"glinides"GLP-1 analogsExenatide • Liraglutide • Taspoglutide† • Albiglutide† • LixisenatideAnalogs/other insulinsfast-acting (Insulin lispro • Insulin aspart • Insulin glulisine) • short-acting (Regular insulin) • long-acting (Insulin glargine • Insulin detemir • NPH insulin) • ultra-long-acting (Insulin degludec†) • inhalable Exubera‡Other Amylin analogSGLT2 inhibitorsCanagliflozin† • Dapagliflozin† • Remogliflozin§ • Sergliflozin§OtherBenfluorex‡ • Tolrestat‡Categories:- Insulin therapies
- Diabetes
- Human proteins
- Recombinant proteins
- Peptide hormones
Wikimedia Foundation. 2010.
