- Hydroxyl radical
-
Hydroxyl radical  Hydroxyl radicalOther namesHydroxy
Hydroxyl radicalOther namesHydroxy
Hydroxyl
λ1-OxidanylIdentifiers CAS number 3352-57-6 PubChem 157350 ChemSpider 138477 
KEGG C16844 
ChEBI CHEBI:29191 Gmelin Reference 105 Jmol-3D images Image 1 - [OH]
Properties Molecular formula HO Molar mass 17.01 g mol−1 Exact mass 17.002739654 g mol-1 Thermochemistry Std enthalpy of
formation ΔfHo29838.99 kJ mol-1 Standard molar
entropy So298183.71 kJ K-1 mol-1 Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references The hydroxyl radical, •OH, is the neutral form of the hydroxide ion (OH–). Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also an important radical formed in radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. Hydroxyl radicals are also produced during UV-light dissociation of H2O2 (suggested in 1879) and likely in Fenton chemistry, where trace amounts of reduced transition metals catalyze peroxide-mediated oxidations of organic compounds.
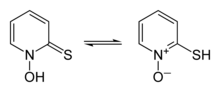
In organic synthesis hydroxyl radicals are most commonly generated by photolysis of 1-Hydroxy-2(1H)-pyridinethione.
The hydroxyl radical is often referred to as the "detergent" of the troposphere because it reacts with many pollutants, often acting as the first step to their removal. It also has an important role in eliminating some greenhouse gases like methane and ozone.[2] The rate of reaction with the hydroxyl radical often determines how long many pollutants last in the atmosphere, if they do not undergo photolysis or are rained out. For instance methane, which reacts relatively slowly with hydroxyl radical, has an average lifetime of >5 years and many CFCs have lifetimes of 50+ years. Pollutants, such as larger hydrocarbons, can have very short average lifetimes of less than a few hours.
The first reaction with many volatile organic compounds (VOCs) is the removal of an hydrogen atom, forming water and an alkyl radical (R•).
- •OH + RH → H2O + R•
The alkyl radical will typically react rapidly with oxygen forming a peroxy radical.
- R• + O2 → RO2
The fate of this radical in the troposphere is dependent on factors such as the amount of sunlight, pollution in the atmosphere and the nature of the alkyl radical that formed it (See chapters 12 & 13 in External Links "University Lecture notes on Atmospheric chemistry)
Contents
- 1 Biological significance
- 2 Importance in the Earth atmosphere
- 3 Astronomical importance
- 4 See also
- 5 Notes
- 6 References
- 7 External links
Biological significance
The hydroxyl radical can damage virtually all types of macromolecules: carbohydrates, nucleic acids (mutations), lipids (lipid peroxidation) and amino acids (e.g. conversion of Phe to m-Tyrosine and o-Tyrosine). PMID 7776173. The hydroxyl radical has a very short in vivo half-life of approximately 10−9 seconds and a high reactivity.[3] This makes it a very dangerous compound to the organism. PMID 7776173. PMID 9288572
Unlike superoxide, which can be detoxified by superoxide dismutase, the hydroxyl radical cannot be eliminated by an enzymatic reaction. Mechanisms for scavenging peroxyl radicals for the protection of cellular structures includes endogenous antioxidants such as melatonin and glutathione, and dietary antioxidants such as mannitol and vitamin E. PMID 7776173.
Importance in the Earth atmosphere
The hydroxyl •OH radicals is one of the main chemical species controlling the oxidizing capacity of the global Earth atmosphere. This oxidizing reactive species has a major impact on the concentrations and distribution of greenhouse gases and pollutants in the Earth atmosphere. It is the most widespread oxidizer in the troposphere, the lowest part of the atmosphere. Understanding •OH variability is important to evaluating human impacts on the atmosphere and climate. The •OH species has a lifetime in the Earth atmosphere of less than one second.[4] Understanding the role of •OH in the oxidation process of methane (CH4) present in the atmosphere is important for assessing the residence time of this greenhouse gas and its influence on the process of global warming. The lifetime of •OH radicals in the Earth atmosphere is very short, therefore •OH concentrations in the air are very low and very sensitive techniques are required for its direct detection.[5] Global average hydroxyl radical concentrations have been measured indirectly by analyzing methyl chloroform (CH3CCl3) present in the air. The results obtained by Montzka et al. (2011)[6] shows that the interannual variability in •OH estimated from CH3CCl3 measurements is small, indicating that global •OH is generally well buffered against perturbations. This small variability is consistent with measurements of methane and other trace gases primarily oxidized by •OH, as well as global photochemical model calculations.
Astronomical importance
First detection of interstellar •OH
The first experimental evidence for the presence of 18-cm absorption lines of the hydroxyl (•OH) radical in the radio absorption spectrum of Cassiopeia A was obtained by Weinreb et al. (Nature, Vol. 200, pp. 829, 1963) based on observations made during the period October 15–29, 1963. [1]
Important subsequent report of •OH astronomical detections
Year Description Reference 1967 •OH Molecules in the Interstellar Medium. Robinson and McGee. One of the first observational reviews of •OH observations. •OH had been observed in absorption and emission, but at this time the processes which populate the energy levels are not yet known with certainty, so the article does not give good estimates of •OH densities. [2] 1967 Normal •OH Emission and Interstellar Dust Clouds. Heiles. First detection of normal emission from •OH in interstellar dust clouds. [3] 1971 Interstellar molecules and dense clouds.D. M. Rank, C. H. Townes, and W. J. Welch. Review of the epoch about molecular line emission of molecules through dense clouds. [4] 1980 •OH observations of molecular complexes in Orion and Taurus. Baud and Wouterloot. Map of •OH emission in molecular complexes Orion and Taurus. Derived column densities are in good agreement with previous CO results. [5] 1981 Emission-absorption observations of OH in diffuse interstellar clouds. Dickey, Crovisier and Kazès. Observations of fifty eight regions which show HI absorption were studied. Typical densities and excitation temperature for diffuse clouds are determined in this article. [6] 1981 Magnetic fields in molecular clouds — •OH Zeeman observations. Crutcher, Troland and Heiles. •OH Zeeman observations of the absorption lines produced in interstellar dust clouds toward 3C 133, 3C 123, and W51. [7] 1981 Detection of interstellar OH in the Far-Infrared. J. Storey , D. Watson, C. Townes. Strong absorption lines of •OH were detected at wavelengths of 119.23 and 119.44 microns in the direction of Sgr B2. [8] 1989 Molecular outflows in powerful OH megamasers. Baan, Haschick and Henkel. Observations of •H and •OH molecular emission through •OH megamasers galaxies, in order to get a FIR luminosity and maser activity relation. [9] Energy levels
•OH is a diatomic molecule. The electronic angular momentum along the molecular axis is +1 or -1, and the electronic spin angular momentum S=1/2. Because of the orbit-spin coupling, the spin angular momentum can be oriented in parallel or anti parallel directions to the orbital angular momentum, producing the splitting into Π1/2 and Π3/2 states. The 2Π3/2 ground state of •OH is split by lambda doubling interaction (an interaction between the nuclei rotation and the unpaired electron motion around its orbit). Hyperfine interaction with the unpaired spin of the proton further splits the levels.
Chemistry of the molecule •OH
In order to study gas phase interstellar chemistry, it is convenient to distinguish two types of interstellar clouds: diffuse clouds, with T=30-100 K, and n=10–1000 cm−3, and dense clouds with T=10-30K and density n=104-103 cm−3. Ion chemical routes in both dense and diffuse clouds have been established for some works (Hartquist 1990).
•OH production pathways
The •OH radical is linked with the production of H2O in molecular clouds. Studies of •OH distribution in Taurus Molecular Cloud-1 (TMC-1) ([10] Harju et al. 2000) suggest that in dense gas, •OH is mainly formed by dissociative recombination of H3O+. Dissociative recombination is the reaction in which a molecular ion recombines with an electron and dissociates into neutral fragments. Important formation mechanisms for •OH are:
H3O+ + e- → •OH + H2 (1a) Dissociative recombination
H3O+ + e- → •OH + •H + •H (1b) Dissociative recombination
HCO2+ + e- → •OH + CO (2a) Dissociative recombination
•O + HCO → •OH + CO (3a) Neutral-neutral
H- + H3O+ → •OH + H2 + •H (4a) Ion-molecular ion neutralization
HCO2 + e- → •OH + CO (5a) Dissociative recombination
•OH destruction pathways
Experimental data on association reactions of •H and •OH suggest that radiative association involving atomic and diatomic neutral radicals may be considered as an effective mechanism for the production of small neutral molecules in the interstellar clouds ([11]Field et al. 1980). The formation of O2 occurs in the gas phase via the neutral exchange reaction between •O and •OH, which is also the main sink for •OH in dense regions ([12] Harju et al. 2000).
We can see that atomic oxygen takes part both in the production and destruction of •OH, so the abundance of •OH depends mainly on the H3+ abundance. Then, important chemical pathways leading from •OH radicals are:
•OH + •O → O2 + •H (1A) Neutral-neutral
•OH + C+ → CO+ + •H (2A) Ion-neutral
•OH + •N → NO + •H (3A) Neutral-neutral
•OH + C → CO + •H (4A) Neutral-neutral
•OH + •H → H2O + photon (5A) Neutral-neutral
Rate constants and relative rates for important formation and destruction mechanisms
Rate constants can be derived from the dataset published in the website [13]. Rate constants have the form:
k(T)=alpha*(T/300)beta*exp(-gamma/T)cm3s−1
The following table has the rate constants calculated for a typical temperature in a dense cloud T=10 K.
Reaction k (T=10 K) cm3s−1 1a 3.29 10−6 1b 1.41 10−7 2a 4.71 10−7 3a 5.0 10−11 4a 1.26 10−6 5a 2.82 10−6 1A 7.7 10−10 2A 3.5 10−11 3A 1.38 10−10 4A 1.0 10−10 5A 3.33 10−14 Formation rates rix can be obtained using the rate constants k(T) and the abundances of the reactants species C and D:
rix=k(T)ix[C][D]
where [Y] represents the abundance of the specie Y. In this approach, abundances were taken from The UMIST database for astrochemistry 2006, and the values are relatives to the H2 density. Following table shows the ratio rix/r1a in order to get a view of the most important reactions.
r1a r1b r2a r3a r4a r5a r1a 1.0 0.043 0.013 0.035 3.6 10−5 0.679 The results suggest that (1a) reaction is the most prominent reaction in dense clouds. It is in concordance with Harju et al. 2000.
Next table shows the results by doing the same procedure for destruction reaction:
r1A r2A r3A r4A r5A r1A 1.0 6.14 10−3 0.152 3.6 10−5 4.29 10−3 Results shows that, 1A reaction is the main sink for OH in dense clouds.
Importance of interstellar •OH observations
Discoveries of the microwave spectra of a considerable number of molecules prove the existence of rather complex molecules in the interstellar clouds, and provides the possibility to study dense clouds, which are obscured by the dust they contain.[7] The •OH molecule has been observed in the interstellar medium since 1963 through its 18-cm transitions.[8] In the subsequent years •OH was observed by its rotational transitions at far infrared wavelengths, mainly in the Orion region. Because each rotational level of •OH is split in by lambda doubling, astronomers can observe a wide variety of energy states from the ground state.
•OH as a tracer of shock conditions
Very high densities are required to thermalize the rotational transitions of •OH,[9] so it is difficult to detect far-infrared emission lines from a quiescent molecular cloud. Even at H2 densities of 106 cm−3, dust must be optically thick at infrared wavelengths. But the passage of a shock wave through a molecular cloud is precisely the process which can bring the molecular gas out of equilibrium with the dust, making observations of far-infrared emission lines possible. A moderately fast shock may produce a transient raise in the •OH abundance relative to hydrogen. So, it is possible that far-infrared emission lines of •OH can be a good diagnostic of shock conditions.
In diffuse clouds
Diffuse clouds are of astronomical interest because they play a primary role in the evolution and thermodynamics of ISM. Observation of the abundant atomic hydrogen in 21 cm has shown good signal-to-noise ratio in both emission and absorption. Nevertheless, HI observations have a fundamental difficulty when are directed to low mass regions of the hydrogen nucleus, as the center part of a diffuse cloud: Thermal width of hydrogen lines are the same order as the internal velocities structures of interest, so clouds components of various temperatures and central velocities are indistinguishable in the spectrum. Molecular lines observations in principle doesn't suffer of this problems. Unlike HI, molecules generally have excitation temperature Tex << Tkin, so that emission is very weak even from abundant species. CO and •OH are the most easily studied candidates molecules. CO has transitions in a region of the spectrum (wavelength < 3 mm) where there is not strong background continuum sources, but •OH has the 18 cm emission, line convenient for absorption observations.[10] Observation studies provide the most sensitive means of detections of molecules with subthermal excitation, and can give the opacity of the spectral line, which is a central issue to model the molecular region.
Studies based in the kinematic comparison of •OH and HI absorption lines from diffuse clouds are useful in determining their physical conditions, specially because heavier elements provide higher velocity resolution.
•OH masers
•OH masers, a type of astrophysical maser, were the first masers to be discovered in space and have been observed in more environments than any other type of maser.
In the Milky Way, •OH masers are found in stellar masers (evolved stars), interstellar masers (regions of massive star formation), or in the interface between supernova remnants and molecular material. Interstellar OH masers are often observed from molecular material surrounding ultracompact H II regions (UC H II). But there are masers associated with very young stars that have yet to create UC H II regions.[11] This class of •OH masers appears to form near the edges of very dense material, place where H2O masers form, and where total densities drop rapidly and UV radiation form young stars can dissociate the H2O molecules. So, observations of •OH masers in these regions, can be an important way to probe the distribution of the important H2O molecule in interstellar shocks at high spacial resolutions.
See also
Notes
- ^ a b "Hydroxyl (CHEBI:29191)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. http://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:29191.
- ^ IPCC assessment report
- ^ Sies, Helmut (March 1993). "Strategies of antioxidant defense". European Journal of Biochemistry 215 (2): 213–219. doi:10.1111/j.1432-1033.1993.tb18025.x. PMID 7688300.
- ^ Isaksen, I.S.A.; S.B. Dalsøren (2011). "Getting a better estimate of an atmospheric radical". Science 331 (6013): 38–39. doi:10.1126/science.1199773. PMID 21212344. http://www.sciencemag.org/content/331/6013/38.short. Retrieved 2011-01-09.
- ^ Heal, M.R.; Heard, D.E.; Pilling, M.J.; Whitaker, B.J. (1995). "On the development and validation of FAGE for local measurement of tropospheric OH and HO2". Journal of Atmospheric Sciences 52 (19): 3428–3448. Bibcode 1995JAtS...52.3428H. doi:10.1175/1520-0469(1995)052<3428:OTDAVO>2.0.CO;2. ISSN 1520-0469.
- ^ Montzka, S.A.; M. Krol, E. Dlugokencky, B. Hall, P. Jöckel, J. Lelieveld (2011). "Small interannual variability of global atmospheric hydroxyl". Science 331 (6013): 67–69. doi:10.1126/science.1197640. PMID 21212353. http://www.sciencemag.org/content/331/6013/67.abstract. Retrieved 2011-01-09.
- ^ Rank, D. M.; Townes, C. H.; Welch, W. J. (1971-12-01). "Interstellar Molecules and Dense Clouds". Science 174 (4014): 1083–1101. doi:10.1126/science.174.4014.1083. PMID 17779392. http://www.sciencemag.org/cgi/content/refs/174/4014/1083. Retrieved 2009-01-13.
- ^ Dieter, N. H.; Ewen, H. I. (1964-01-18). "Radio Observations of the Interstellar OH Line at 1,667 Mc/s". Nature 201 (4916): 279–281. doi:10.1038/201279b0. http://www.nature.com/nature/journal/v201/n4916/abs/201279b0.html. Retrieved 2009-01-13.
- ^ Storey, J. W. V.; Watson, D. M.; Townes, C. H. (1981-02-15). "Detection of interstellar OH in the far-infrared". Astrophysical Journal, Part 2 - Letters to the Editor 244: L27–L30. Bibcode 1981ApJ...244L..27S. doi:10.1086/183472.
- ^ Dickey, J. M.; Crovisier, J.; Kazes, I. (May 1981). "Emission-absorption observations of •OH in diffuse interstellar clouds". Astronomy and Astrophysics 98 (2): 271–285. Bibcode 1981A&A....98..271D.
- ^ Argon, Alice L.; Reid, Mark J.; Menten, Karl M. (August 2003). "A class of interstellar •OH masers associated with protostellar outflows". The Astrophysical Journal 593 (2): 925–930. Bibcode 2003ApJ...593..925A. doi:10.1086/376592.
References
- Downes, A. and Blunt, T.P. (1879). The effect of sunlight upon hydrogen peroxide. Nature 20, 521.
External links
Categories:- Alcohols
- Biological processes
- Environmental chemistry
- Free radicals
- Hydroxides
- Reactive intermediates
Wikimedia Foundation. 2010.