- CD4
-
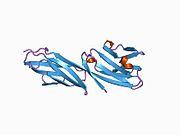
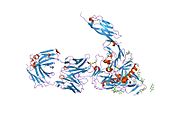
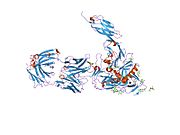
CD4, extracellular 
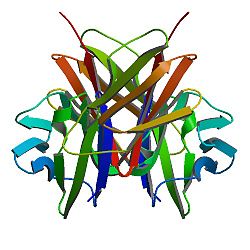
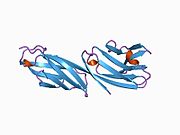
structure of t-cell surface glycoprotein cd4, monoclinic crystal form Identifiers Symbol CD4-extracel Pfam PF09191 InterPro IPR015274 SCOP 1cid Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CD4 (cluster of differentiation 4) is a glycoprotein expressed on the surface of T helper cells, monocytes, macrophages, and dendritic cells. It was discovered in the late 1970s and was originally known as leu-3 and T4 (after the OKT4 monoclonal antibody that reacted with it) before being named CD4 in 1984.[2] In humans, the CD4 protein is encoded by the CD4 gene.[3][4]
Contents
Structure
Like many cell surface receptors/markers, CD4 is a member of the immunoglobulin superfamily.
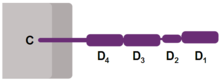
It has four immunoglobulin domains (D1 to D4) that are exposed on the extracellular surface of the cell:
- D1 and D3 resemble immunoglobulin variable (IgV) domains.
- D2 and D4 resemble immunoglobulin constant (IgC) domains.
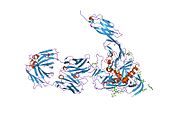
CD4 uses its D1 domain to interact with the β2-domain of MHC class II molecules. T cells expressing CD4 molecules (and not CD8) on their surface, therefore, are specific for antigens presented by MHC II and not by MHC class I (they are MHC class II-restricted).
The short cytoplasmic/intracellular tail (C) of CD4 contains a special sequence of amino acids that allow it to interact with the lck molecule described above.
Function
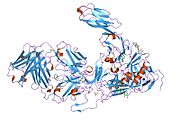
CD4 is a co-receptor that assists the T cell receptor (TCR) with an antigen-presenting cell. Using its portion that resides inside the T cell, CD4 amplifies the signal generated by the TCR by recruiting an enzyme, known as the tyrosine kinase lck, which is essential for activating many molecules involved in the signaling cascade of an activated T cell. CD4 also interacts directly with MHC class II molecules on the surface of the antigen-presenting cell using its extracellular domain. The extracellular domain adopts an immunoglobulin-like beta-sandwich with seven strands in 2 beta sheets, in a Greek key topology.[5]
Other Interactions
CD4 has also been shown to interact with SPG21,[6] Lck[7][8][9][10][11] and Protein unc-119 homolog.[12]
Disease
HIV infection
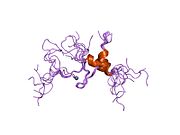
HIV-1 uses CD4 to gain entry into host T-cells and achieves this by binding of the viral envelope protein known as gp120 to CD4.[13] The binding to CD4 creates a shift in the conformation of gp120 allowing HIV-1 to bind to a co-receptor expressed on the host cell. These co-receptors are chemokine receptors CCR5 or CXCR4, which of these co-receptor is used during infection is dependent on whether the virus is infecting a macrophage or T-helper cell[citation needed]. Following a structural change in another viral protein (gp41), HIV inserts a fusion peptide into the host cell that allows the outer membrane of the virus to fuse with the cell membrane.
HIV pathology
HIV infection leads to a progressive reduction in the number of T cells expressing CD4. Medical professionals refer to the CD4 count to decide when to begin treatment during HIV infection. Normal blood values are 500-1200x10-6/L.[14] CD4 count test measures the number of T cells expressing CD4. Results are usually expressed in the number of cells per microliter (or cubic millimeter, mm3) of blood. While CD4 tests are not a direct HIV test e.g. does not check the presence of viral DNA, or specific antibodies against HIV, the CD4 count is used to assess the immune system of patients. Patients often undergo treatments when the CD4 count reach a level of 350 cells per microliter; less than 200 cells per microliter in an HIV positive individual is diagnosed as AIDS. Medical professionals also refer to CD4 tests to determine efficacy of treatment.
 Reference ranges for blood tests of white blood cells, comparing CD4+ cell amount (shown in green-yellow) with other cells.
Reference ranges for blood tests of white blood cells, comparing CD4+ cell amount (shown in green-yellow) with other cells.
Other diseases
CD4 continues to be expressed in most neoplasms derived from T helper cells. It is therefore possible to use CD4 immunohistochemistry on tissue biopsy samples to identify most forms of peripheral T cell lymphoma and related malignant conditions.[15] The antigen has also been associated with a number of autoimmune diseases such as vitiligo and type I diabetes mellitus.[16]
References
- ^ PDB 1cdh; Ryu SE, Truneh A, Sweet RW, Hendrickson WA (January 1994). "Structures of an HIV and MHC binding fragment from human CD4 as refined in two crystal lattices". Structure 2 (1): 59–74. PMID 8075984.
- ^ Alain Bernard (1984). Leucocyte typing: human leucocyte differentiation antigens detected by monoclonal antibodies: specification, classification, nomenclature: [report on the first international references workshop sponsored by INSERM, WHO and IUIS]. Berlin: Springer. pp. pages 45–48. ISBN 0-387-12056-4.
- ^ Isobe M, Huebner K, Maddon PJ, Littman DR, Axel R, Croce CM (June 1986). "The gene encoding the T-cell surface protein T4 is located on human chromosome 12". Proc. Natl. Acad. Sci. U.S.A. 83 (12): 4399–402. doi:10.1073/pnas.83.12.4399. PMC 323740. PMID 3086883. http://www.pnas.org/cgi/pmidlookup?view=long&pmid=3086883.
- ^ Ansari-Lari MA, Muzny DM, Lu J, Lu F, Lilley CE, Spanos S, Malley T, Gibbs RA (April 1996). "A gene-rich cluster between the CD4 and triosephosphate isomerase genes at human chromosome 12p13". Genome Res. 6 (4): 314–26. doi:10.1101/gr.6.4.314. PMID 8723724. http://www.genome.org/cgi/pmidlookup?view=long&pmid=8723724.
- ^ Brady RL, Dodson EJ, Dodson GG, Lange G, Davis SJ, Williams AF, Barclay AN (May 1993). "Crystal structure of domains 3 and 4 of rat CD4: relation to the NH2-terminal domains". Science 260 (5110): 979–83. doi:10.1126/science.8493535. PMID 8493535.
- ^ Zeitlmann, L; Sirim P, Kremmer E, Kolanus W (Mar. 2001). "Cloning of ACP33 as a novel intracellular ligand of CD4". J. Biol. Chem. (United States) 276 (12): 9123–32. doi:10.1074/jbc.M009270200. ISSN 0021-9258. PMID 11113139.
- ^ Rudd CE, Trevillyan JM, Dasgupta JD, Wong LL, Schlossman SF (September 2010). "Pillars article: the CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. 1988". J. Immunol. 185 (5): 2645–9. PMID 20724730.
- ^ Rudd CE, Trevillyan JM, Dasgupta JD, Wong LL, Schlossman SF (July 1988). "The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes". Proc. Natl. Acad. Sci. U.S.A. 85 (14): 5190–4. doi:10.1073/pnas.85.14.5190. PMC 281714. PMID 2455897. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=281714.
- ^ Barber EK, Dasgupta JD, Schlossman SF, Trevillyan JM, Rudd CE (May 1989). "The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex". Proc. Natl. Acad. Sci. U.S.A. 86 (9): 3277–81. doi:10.1073/pnas.86.9.3277. PMC 287114. PMID 2470098. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=287114.
- ^ Hawash IY, Hu XE, Adal A, Cassady JM, Geahlen RL, Harrison ML (April 2002). "The oxygen-substituted palmitic acid analogue, 13-oxypalmitic acid, inhibits Lck localization to lipid rafts and T cell signaling". Biochim. Biophys. Acta 1589 (2): 140–50. doi:10.1016/S0167-4889(02)00165-9. PMID 12007789.
- ^ Foti M, Phelouzat MA, Holm A, Rasmusson BJ, Carpentier JL (February 2002). "p56Lck anchors CD4 to distinct microdomains on microvilli". Proc. Natl. Acad. Sci. U.S.A. 99 (4): 2008–13. doi:10.1073/pnas.042689099. PMC 122310. PMID 11854499. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=122310.
- ^ Gorska MM, Stafford SJ, Cen O, Sur S, Alam R (February 2004). "Unc119, a novel activator of Lck/Fyn, is essential for T cell activation". J. Exp. Med. 199 (3): 369–79. doi:10.1084/jem.20030589. PMC 2211793. PMID 14757743. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2211793.
- ^ Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA (June 1998). "Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody". Nature 393 (6686): 648–59. doi:10.1038/31405. PMID 9641677.
- ^ Bofill M, Janossy G, Lee CA, MacDonald-Burns D, Phillips AN, Sabin C, Timms A, Johnson MA, Kernoff PB (May 1992). "Laboratory control values for CD4 and CD8 T lymphocytes. Implications for HIV-1 diagnosis". Clin. Exp. Immunol. 88 (2): 243–52. PMC 1554313. PMID 1349272. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1554313.
- ^ Kumarasen Cooper; Anthony S-Y. Leong (2003). Manual of diagnostic antibodies for immunohistology. London: Greenwich Medical Media. pp. 65. ISBN 1-84110-100-1.
- ^ Zamani M, Tabatabaiefar MA, Mosayyebi S, Mashaghi A, Mansouri P (July 2010). "Possible association of the CD4 gene polymorphism with vitiligo in an Iranian population". Clin. Exp. Dermatol. 35 (5): 521–4. doi:10.1111/j.1365-2230.2009.03667.x. PMID 19843086.
Further reading
- Miceli MC, Parnes JR (1993). "Role of CD4 and CD8 in T cell activation and differentiation". Adv. Immunol. 53: 59–122. doi:10.1016/S0065-2776(08)60498-8. PMID 8512039.
- Geyer M, Fackler OT, Peterlin BM (2001). "Structure--function relationships in HIV-1 Nef". EMBO Rep. 2 (7): 580–5. doi:10.1093/embo-reports/kve141. PMC 1083955. PMID 11463741. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1083955.
- Greenway AL, Holloway G, McPhee DA, et al. (2004). "HIV-1 Nef control of cell signalling molecules: multiple strategies to promote virus replication". J. Biosci. 28 (3): 323–35. doi:10.1007/BF02970151. PMID 12734410.
- Bénichou S, Benmerah A (2003). "[The HIV nef and the Kaposi-sarcoma-associated virus K3/K5 proteins: "parasites"of the endocytosis pathway]". Med Sci (Paris) 19 (1): 100–6. doi:10.1051/medsci/2003191100. PMID 12836198.
- Leavitt SA, SchOn A, Klein JC, et al. (2004). "Interactions of HIV-1 proteins gp120 and Nef with cellular partners define a novel allosteric paradigm". Curr. Protein Pept. Sci. 5 (1): 1–8. doi:10.2174/1389203043486955. PMID 14965316.
- Tolstrup M, Ostergaard L, Laursen AL, et al. (2004). "HIV/SIV escape from immune surveillance: focus on Nef". Curr. HIV Res. 2 (2): 141–51. doi:10.2174/1570162043484924. PMID 15078178.
- Hout DR, Mulcahy ER, Pacyniak E, et al. (2005). "Vpu: a multifunctional protein that enhances the pathogenesis of human immunodeficiency virus type 1". Curr. HIV Res. 2 (3): 255–70. doi:10.2174/1570162043351246. PMID 15279589.
- Joseph AM, Kumar M, Mitra D (2005). "Nef: "necessary and enforcing factor" in HIV infection". Curr. HIV Res. 3 (1): 87–94. doi:10.2174/1570162052773013. PMID 15638726.
- Anderson JL, Hope TJ (2005). "HIV accessory proteins and surviving the host cell". Current HIV/AIDS reports 1 (1): 47–53. doi:10.1007/s11904-004-0007-x. PMID 16091223.
- Li L, Li HS, Pa, et al. (2006). "Roles of HIV-1 auxiliary proteins in viral pathogenesis and host-pathogen interactions". Cell Res. 15 (11-12): 923–34. doi:10.1038/sj.cr.7290370. PMID 16354571.
- Stove V, Verhasselt B (2006). "Modelling thymic HIV-1 Nef effects". Curr. HIV Res. 4 (1): 57–64. doi:10.2174/157016206775197583. PMID 16454711.
External links
- MeSH CD1+Antigen
- Mouse CD Antigen Chart
- Human CD Antigen Chart
- *Human Immunodeficiency Virus Glycoprotein 120
PDB gallery 1cdh: STRUCTURES OF AN HIV AND MHC BINDING FRAGMENT FROM HUMAN CD4 AS REFINED IN TWO CRYSTAL LATTICES1cdi: STRUCTURES OF AN HIV AND MHC BINDING FRAGMENT FROM HUMAN CD4 AS REFINED IN TWO CRYSTAL LATTICES1cdj: STRUCTURE OF T-CELL SURFACE GLYCOPROTEIN CD41cdu: STRUCTURE OF T-CELL SURFACE GLYCOPROTEIN CD4 MUTANT WITH PHE 43 REPLACED BY VAL1cdy: STRUCTURE OF T-CELL SURFACE GLYCOPROTEIN CD4 MUTANT WITH GLY 47 REPLACED BY SER1g9m: HIV-1 HXBC2 GP120 ENVELOPE GLYCOPROTEIN COMPLEXED WITH CD4 AND INDUCED NEUTRALIZING ANTIBODY 17B1g9n: HIV-1 YU2 GP120 ENVELOPE GLYCOPROTEIN COMPLEXED WITH CD4 AND INDUCED NEUTRALIZING ANTIBODY 17B1gc1: HIV-1 GP120 CORE COMPLEXED WITH CD4 AND A NEUTRALIZING HUMAN ANTIBODY1jl4: CRYSTAL STRUCTURE OF THE HUMAN CD4 N-TERMINAL TWO DOMAIN FRAGMENT COMPLEXED TO A CLASS II MHC MOLECULE1q68: Solution structure of T-cell surface glycoprotein CD4 and Proto-oncogene tyrosine-protein kinase LCK fragments1rzj: HIV-1 HXBC2 GP120 ENVELOPE GLYCOPROTEIN COMPLEXED WITH CD4 AND INDUCED NEUTRALIZING ANTIBODY 17B1rzk: HIV-1 YU2 GP120 ENVELOPE GLYCOPROTEIN COMPLEXED WITH CD4 AND INDUCED NEUTRALIZING ANTIBODY 17B1wio: STRUCTURE OF T-CELL SURFACE GLYCOPROTEIN CD4, TETRAGONAL CRYSTAL FORM1wip: STRUCTURE OF T-CELL SURFACE GLYCOPROTEIN CD4, MONOCLINIC CRYSTAL FORM1wiq: STRUCTURE OF T-CELL SURFACE GLYCOPROTEIN CD4, TRIGONAL CRYSTAL FORM2b4c: Crystal structure of HIV-1 JR-FL gp120 core protein containing the third variable region (V3) complexed with CD4 and the X5 antibody2nxy: HIV-1 gp120 Envelope Glycoprotein(S334A) Complexed with CD4 and Antibody 17b2nxz: HIV-1 gp120 Envelope Glycoprotein (T257S, S334A, S375W) Complexed with CD4 and Antibody 17b2ny0: HIV-1 gp120 Envelope Glycoprotein (M95W, W96C, T257S, V275C, S334A, S375W, A433M) Complexed with CD4 and Antibody 17b2ny1: HIV-1 gp120 Envelope Glycoprotein (I109C, T257S, S334A, S375W, Q428C) Complexed with CD4 and Antibody 17b2ny2: HIV-1 gp120 Envelope Glycoprotein (T123C, T257S, S334A, S375W, G431C) Complexed with CD4 and Antibody 17b2ny3: HIV-1 gp120 Envelope Glycoprotein (K231C, T257S, E267C, S334A, S375W) Complexed with CD4 and Antibody 17b2ny4: HIV-1 gp120 Envelope Glycoprotein (K231C, T257S, E268C, S334A, S375W) Complexed with CD4 and Antibody 17b2ny5: HIV-1 gp120 Envelope Glycoprotein (M95W, W96C, I109C, T257S, V275C, S334A, S375W, Q428C, A433M) Complexed with CD4 and Antibody 17b2ny6: HIV-1 gp120 Envelope Glycoprotein (M95W, W96C, I109C, T123C, T257S, V275C,S334A, S375W, Q428C, G431C) Complexed with CD4 and Antibody 17b3cd4: REFINEMENT AND ANALYSIS OF THE FIRST TWO DOMAINS OF HUMAN CD4Transmembrane receptors: Immunoglobulin superfamily immune receptors Antibody receptor:
Fc receptorSecretoryAntigen receptor Antigen receptorAccessory moleculesT cellsAntigen receptorAccessory moleculesCytokine receptor see cytokine receptorsKiller-cell IG-like receptors Leukocyte IG-like receptors B trdu: iter (nrpl/grfl/cytl/horl), csrc (lgic, enzr, gprc, igsr, intg, nrpr/grfr/cytr), itra (adap, gbpr, mapk), calc, lipd; path (hedp, wntp, tgfp+mapp, notp, jakp, fsap, hipp, tlrp)1-50 CD1 (a-c, 1A, 1D, 1E) · CD2 · CD3 (γ, δ, ε) · CD4 · CD5 · CD6 · CD7 · CD8 (a) · CD9 · CD10 · CD11 (a, b, c) · CD13 · CD14 · CD15 · CD16 (A, B) · CD18 · CD19 · CD20 · CD21 · CD22 · CD23 · CD24 · CD25 · CD26 · CD27 · CD28 · CD29 · CD30 · CD31 · CD32 (A, B) · CD33 · CD34 · CD35 · CD36 · CD37 · CD38 · CD39 · CD40 · CD41 · CD42 (a, b, c, d) · CD43 · CD44 · CD45 · CD46 · CD47 · CD48 · CD49 (a, b, c, d, e, f) · CD5051-100 CD51 · CD52 · CD53 · CD54 · CD55 · CD56 · CD57 · CD58 · CD59 · CD61 · CD62 (E, L, P) · CD63 · CD64 (A, B, C) · CD66 (a, b, c, d, e, f) · CD68 · CD69 · CD70 · CD71 · CD72 · CD73 · CD74 · CD78 · CD79 (a, b) · CD80 · CD81 · CD82 · CD83 · CD84 · CD85 (a, d, e, h, j, k) · CD86 · CD87 · CD88 · CD89 · CD90 · CD91- CD92 · CD93 · CD94 · CD95 · CD96 · CD97 · CD98 · CD99 · CD100101-150 CD101 · CD102 · CD103 · CD104 · CD105 · CD106 · CD107 (a, b) · CD108 · CD109 · CD110 · CD111 · CD112 · CD113 · CD114 · CD115 · CD116 · CD117 · CD118 · CD119 · CD120 (a, b) · CD121 (a, b) · CD122 · CD123 · CD124 · CD125 · CD126 · CD127 · CD129 · CD130 · CD131 · CD132 · CD133 · CD134 · CD135 · CD136 · CD137 · CD138 · CD140b · CD141 · CD142 · CD143 · CD144 · CD146 · CD147 · CD148 · CD150151-200 CD151 · CD152 · CD153 · CD154 · CD155 · CD156 (a, b, c) · CD157 · CD158 (a, d, e, i, k) · CD159 (a, c) · CD160 · CD161 · CD162 · CD163 · CD164 · CD166 · CD167 (a, b) · CD168 · CD169 · CD170 · CD171 · CD172 (a, b, g) · CD174 · CD177 · CD178 · CD179 (a, b) · CD181 · CD182 · CD183 · CD184 · CD185 · CD186 · CD191 · CD192 · CD193 · CD194 · CD195 · CD196 · CD197 · CDw198 · CDw199 · CD200201-250 CD201 · CD202b · CD204 · CD205 · CD206 · CD207 · CD208 · CD209 · CDw210 (a, b) · CD212 · CD213a (1, 2) · CD217 · CD218 (a, b) · CD220 · CD221 · CD222 · CD223 · CD224 · CD225 · CD226 · CD227 · CD228 · CD229 · CD230 · CD233 · CD234 · CD235 (a, b) · CD236 · CD238 · CD239 · CD240CE · CD240D · CD241 · CD243 · CD244 · CD246 · CD247- CD248 · CD249251-300 CD252 · CD253 · CD254 · CD256 · CD257 · CD258 · CD261 · CD262 · CD264 · CD265 · CD266 · CD267 · CD268 · CD269 · CD271 · CD272 · CD273 · CD274 · CD275 · CD276 · CD278 · CD279 · CD280 · CD281 · CD282 · CD283 · CD284 · CD286 · CD288 · CD289 · CD290 · CD292 · CDw293 · CD294 · CD295 · CD297 · CD298 · CD299301-350 Lymphoid Pre-B cell: CD10/CALLA · CD79A
mature: CD19 · CD20 · CD21/CR2 · CD23/FcεRII · CD127 · CD40
plasma cell: CD38 · CD138T/NKNK cellAllAllMyeloid CFU-MegCFU-EAll (pan-myeloid)Stem cell This article includes text from the public domain Pfam and InterPro IPR015274
Categories:- Human proteins
- Protein domains
- Immunology
- Glycoproteins
- Clusters of differentiation
- T cell
- Dendritic cell
Wikimedia Foundation. 2010.