- Band 3
-
solute carrier family 4 (anion exchanger), member 1, adapter protein 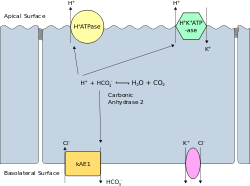
Identifiers Symbol SLC4A1AP Entrez 22950 HUGO 13813 OMIM 602655 RefSeq NM_018158 UniProt Q9BWU0 Other data Locus Chr. 2 p23.3 Anion Exchanger 1 (AE1) or Band 3 is a phylogenetically preserved transport protein responsible for mediating the exchange of chloride (Cl-) for bicarbonate (HCO3-) across a plasma membrane. Functionally similar members of the AE clade are AE2 and AE3.[1]
It is ubiquitous throughout the vertebrates. In humans it is present in two specific sites:
- the erythrocyte (red blood cell) cell membrane and
- the basolateral surface of the alpha-intercalated cell (the acid secreting cell type) in the collecting duct of the kidney.
The erythrocyte and kidney forms are different isoforms of the same protein.
Contents
Discovery
AE1 was discovered following SDS-PAGE gel electrophoresis of erythrocyte cell membrane. The large 'third' band on the electrophoresis gel represented AE1, which was thus initially termed 'Band 3'. The chloride-bicarbonate exchanger in the red cell membrane is not a pump, which would use metabolic energy. Nor is it strictly an enzyme. It is protein counter-transporter, known as band III.[2]
AE1 in Red Cells
AE1 is an important structural component of the erythrocyte cell membrane, making up to 25% of the cell membrane surface. Each red cell contains approximately one million copies of AE1.
Function
Here it performs two functions:
- Electroneutral chloride and bicarbonate exchange across the plasma membrane on a one-for-one basis.This is crucial for CO2 uptake by the red cell and conversion (by hydration catalysed by carbonic anhydrase) into a proton and a bicarbonate ion. The bicarbonate is then extruded from the cell by the band 3 molecule.
- Physical linkage of the plasma membrane to the underlying membrane skeleton (via binding with ankyrin and protein 4.2). This appears to be to prevent membrane surface loss, rather than being to do with membrane skeleton assembly.
Pathology
Mutations of erythroid AE1 affecting the extracellular domains of the molecule may cause alterations in the individual's blood group, as band 3 determines the Diego blood group.
More importantly erythroid AE1 mutations cause between 15-25% of cases of Hereditary spherocytosis (a disorder associated with progressive red cell membrane loss), and also cause the hereditary conditions of Hereditary stomatocytosis [3] and Southeast Asian Ovalocytosis [4]
AE1 in Alpha-Intercalated cells
A different isoform of AE1, known as kAE1 (which is 65 amino acids shorter than erythroid AE1) is found in the basolateral surface of the alpha-intercalated cell in the cortical collecting duct of the kidney.
Function
This is the principal acid secreting cell of the kidney, which generates hydrogen ions and bicarbonate ions from carbon dioxide and water-a reaction catalysed by Carbonic anhydrase.
The hydrogen ions are pumped into the collecting duct tubule by vacuolar H+ATPase, the apical proton pump,which thus excretes acid into the urine.
kAE1 exchanges bicarbonate for chloride on the basolateral surface, essentially returning bicarbonate to the blood.
Pathology
Mutations of kidney AE1 cause distal (type1) renal tubular acidosis, which is an inability to acidify the urine, even if the blood is too acid. These mutations are disease causing as they cause mistargetting of the mutant band 3 proteins so that they are retained within the cell or occasionally addressed to the wrong (i.e. apical) surface.
Interactions
Band 3 has been shown to interact with Carbonic anhydrase II[5][6][7][8] and CA4.[9]
See also
References
- ^ Alper, S. L. (2009). "Molecular physiology and genetics of Na+-independent SLC4 anion exchangers". Journal of Experimental Biology 212 (11): 1672–1683. doi:10.1242/jeb.029454. PMC 2683012. PMID 19448077. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2683012.
- ^ Hunter M (1977). "Human erythrocyte anion permeabilities measured under conditions of net charge transfer". J Physiol 268 (1): 35–49. PMC 1283651. PMID 874904. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1283651.
- ^ LJ Bruce; Robinson, HC; Guizouarn, H; Borgese, F; Harrison, P; King, MJ; Goede, JS; Coles, SE et al. (November 2005). "Monovalent cation leaks in human red cells caused by single amino-acid substitutions in the transport domain of the band 3 chloride-bicarbonate exchanger, AE1". Nature Genetics 37 (11): 1258–63. doi:10.1038/ng1656. PMID 16227998.
- ^ Jarolim p; Palek, J; Amato, D; Hassan, K; Sapak, P; Nurse, GT; Rubin, HL; Zhai, S et al. (1991). "Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis". PNAS 88 (24): 11022–11026. Bibcode 1991PNAS...8811022J. doi:10.1073/pnas.88.24.11022. PMC 53065. PMID 1722314. http://www.pnas.org/cgi/content/abstract/88/24/11022.
- ^ Sterling, D; Reithmeier R A, Casey J R (Dec. 2001). "A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers". J. Biol. Chem. (United States) 276 (51): 47886–94. doi:10.1074/jbc.M105959200. ISSN 0021-9258. PMID 11606574.
- ^ Vince, J W; Reithmeier R A (Oct. 1998). "Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1-/HCO3- exchanger". J. Biol. Chem. (UNITED STATES) 273 (43): 28430–7. doi:10.1074/jbc.273.43.28430. ISSN 0021-9258. PMID 9774471.
- ^ Vince, J W; Carlsson U, Reithmeier R A (Nov. 2000). "Localization of the Cl-/HCO3- anion exchanger binding site to the amino-terminal region of carbonic anhydrase II". Biochemistry (UNITED STATES) 39 (44): 13344–9. doi:10.1021/bi0015111. ISSN 0006-2960. PMID 11063570.
- ^ Vince, J W; Reithmeier R A (May. 2000). "Identification of the carbonic anhydrase II binding site in the Cl(-)/HCO(3)(-) anion exchanger AE1". Biochemistry (UNITED STATES) 39 (18): 5527–33. doi:10.1021/bi992564p. ISSN 0006-2960. PMID 10820026.
- ^ Sterling, Deborah; Alvarez Bernardo V, Casey Joseph R (Jul. 2002). "The extracellular component of a transport metabolon. Extracellular loop 4 of the human AE1 Cl-/HCO3- exchanger binds carbonic anhydrase IV". J. Biol. Chem. (United States) 277 (28): 25239–46. doi:10.1074/jbc.M202562200. ISSN 0021-9258. PMID 11994299.
External links
- Diego blood group system at BGMUT Blood Group Antigen Gene Mutation Database at NCBI, NIH
- MeSH Band+3+Protein
- MeSH Chloride-Bicarbonate+Antiporters
Further reading
- Tanner MJ (1993). "Molecular and cellular biology of the erythrocyte anion exchanger (AE1)". Semin. Hematol. 30 (1): 34–57. PMID 8434259.
- Chambers EJ, Askin D, Bloomberg GB, et al. (1998). "Studies on the structure of a transmembrane region and a cytoplasmic loop of the human red cell anion exchanger (band 3, AE1)". Biochem. Soc. Trans. 26 (3): 516–20. PMID 9765907.
- Inaba M (2002). "[Band 3: expanding knowledge on its functions]". Seikagaku 73 (12): 1431–5. PMID 11831035.
- Tanner MJ (2002). "Band 3 anion exchanger and its involvement in erythrocyte and kidney disorders". Curr. Opin. Hematol. 9 (2): 133–9. doi:10.1097/00062752-200203000-00009. PMID 11844997.
- Shayakul C, Alper SL (2004). "Defects in processing and trafficking of the AE1 Cl-/HCO3- exchanger associated with inherited distal renal tubular acidosis". Clin. Exp. Nephrol. 8 (1): 1–11. doi:10.1007/s10157-003-0271-x. PMID 15067510.
PDB gallery 1bh7: A LOW ENERGY STRUCTURE FOR THE FINAL CYTOPLASMIC LOOP OF BAND 3, NMR, MINIMIZED AVERAGE STRUCTURE1bzk: STRUCTURAL STUDIES ON THE EFFECTS OF THE DELETION IN THE RED CELL ANION EXCHANGER (BAND3, AE1) ASSOCIATED WITH SOUTH EAST ASIAN OVALOCYTOSIS.1hyn: CRYSTAL STRUCTURE OF THE CYTOPLASMIC DOMAIN OF HUMAN ERYTHROCYTE BAND-3 PROTEIN1-50 CD1 (a-c, 1A, 1D, 1E) · CD2 · CD3 (γ, δ, ε) · CD4 · CD5 · CD6 · CD7 · CD8 (a) · CD9 · CD10 · CD11 (a, b, c) · CD13 · CD14 · CD15 · CD16 (A, B) · CD18 · CD19 · CD20 · CD21 · CD22 · CD23 · CD24 · CD25 · CD26 · CD27 · CD28 · CD29 · CD30 · CD31 · CD32 (A, B) · CD33 · CD34 · CD35 · CD36 · CD37 · CD38 · CD39 · CD40 · CD41 · CD42 (a, b, c, d) · CD43 · CD44 · CD45 · CD46 · CD47 · CD48 · CD49 (a, b, c, d, e, f) · CD5051-100 CD51 · CD52 · CD53 · CD54 · CD55 · CD56 · CD57 · CD58 · CD59 · CD61 · CD62 (E, L, P) · CD63 · CD64 (A, B, C) · CD66 (a, b, c, d, e, f) · CD68 · CD69 · CD70 · CD71 · CD72 · CD73 · CD74 · CD78 · CD79 (a, b) · CD80 · CD81 · CD82 · CD83 · CD84 · CD85 (a, d, e, h, j, k) · CD86 · CD87 · CD88 · CD89 · CD90 · CD91- CD92 · CD93 · CD94 · CD95 · CD96 · CD97 · CD98 · CD99 · CD100101-150 CD101 · CD102 · CD103 · CD104 · CD105 · CD106 · CD107 (a, b) · CD108 · CD109 · CD110 · CD111 · CD112 · CD113 · CD114 · CD115 · CD116 · CD117 · CD118 · CD119 · CD120 (a, b) · CD121 (a, b) · CD122 · CD123 · CD124 · CD125 · CD126 · CD127 · CD129 · CD130 · CD131 · CD132 · CD133 · CD134 · CD135 · CD136 · CD137 · CD138 · CD140b · CD141 · CD142 · CD143 · CD144 · CD146 · CD147 · CD148 · CD150151-200 CD151 · CD152 · CD153 · CD154 · CD155 · CD156 (a, b, c) · CD157 · CD158 (a, d, e, i, k) · CD159 (a, c) · CD160 · CD161 · CD162 · CD163 · CD164 · CD166 · CD167 (a, b) · CD168 · CD169 · CD170 · CD171 · CD172 (a, b, g) · CD174 · CD177 · CD178 · CD179 (a, b) · CD181 · CD182 · CD183 · CD184 · CD185 · CD186 · CD191 · CD192 · CD193 · CD194 · CD195 · CD196 · CD197 · CDw198 · CDw199 · CD200201-250 CD201 · CD202b · CD204 · CD205 · CD206 · CD207 · CD208 · CD209 · CDw210 (a, b) · CD212 · CD213a (1, 2) · CD217 · CD218 (a, b) · CD220 · CD221 · CD222 · CD223 · CD224 · CD225 · CD226 · CD227 · CD228 · CD229 · CD230 · CD233 · CD234 · CD235 (a, b) · CD236 · CD238 · CD239 · CD240CE · CD240D · CD241 · CD243 · CD244 · CD246 · CD247- CD248 · CD249251-300 CD252 · CD253 · CD254 · CD256 · CD257 · CD258 · CD261 · CD262 · CD264 · CD265 · CD266 · CD267 · CD268 · CD269 · CD271 · CD272 · CD273 · CD274 · CD275 · CD276 · CD278 · CD279 · CD280 · CD281 · CD282 · CD283 · CD284 · CD286 · CD288 · CD289 · CD290 · CD292 · CDw293 · CD294 · CD295 · CD297 · CD298 · CD299301-350 F- and V-type ATPase (3.A.2) P-type ATPase (3.A.3) - 3.A.3.1.1: Na+/K+ transporting: ATP1A1, ATP1A2, ATP1A3, ATP1A4, ATP1B1, ATP1B2, ATP1B3, ATP1B4, ATP1G1
- 3.A.3.1.2: H+/K+, H+/K+ exchanging: ATP4A, ATP4B
- 3.A.3.1.4: H+/K+ transporting, nongastric: ATP12A
- 3.A.3.2: Ca+ (SERCA, PMCA, SPCA) / Ca++ transporting: ATP2A1, ATP2A2, ATP2A3, ATP2B1, ATP2B2, ATP2B3, ATP2B4, ATP2C1
- 3.A.3.8.8: flippase: ATP8A2
Other/ungrouped:
see also ATPase disorders
B memb: cead, trns (1A, 1C, 1F, 2A, 3A1, 3A2-3, 3D), othrBy group SLC1–10 - (6) sodium- and chloride- dependent sodium:neurotransmitter symporters (SLC6A1, SLC6A2, SLC6A3, SLC6A4, SLC6A5, SLC6A6, SLC6A7, SLC6A8, SLC6A9, SLC6A10, SLC6A11, SLC6A12, SLC6A13, SLC6A14, SLC6A15, SLC6A16, SLC6A17, SLC6A18, SLC6A19, SLC6A20)
- (7) cationic amino-acid transporter/glycoprotein-associated (SLC7A1, SLC7A2, SLC7A3, SLC7A4) glycoprotein-associated/light or catalytic subunits of heterodimeric amino-acid transporters (SLC7A5, SLC7A6, SLC7A7, SLC7A8, SLC7A9, SLC7A10, SLC7A11, SLC7A13, SLC7A14)
- (8) Na+/Ca2+ exchanger (SLC8A1, SLC8A2, SLC8A3)
SLC11–20 - (12) electroneutral cation-Cl cotransporter (SLC12A1, SLC12A1, SLC12A2, SLC12A3, SLC12A4, SLC12A5, SLC12A6, SLC12A7, SLC12A8, SLC12A9)
- (14) urea transporter (SLC14A1, SLC14A2)
- (15) proton oligopeptide cotransporter (SLC15A1, SLC15A2, SLC15A3, SLC15A4)
- (16) monocarboxylate transporter (SLC16A1, SLC16A2, SLC16A3, SLC16A4, SLC16A5, SLC16A6, SLC16A7, SLC16A8, SLC16A9, SLC16A10, SLC16A11, SLC16A12, SLC16A13, SLC16A14)
SLC21–30 - (21) organic anion transporting (SLCO1A2, SLCO1B1, SLCO1B3, SLCO1B4, SLCO1C1) (SLCO2A1, SLCO2B1) (SLCO3A1) (SLCO4A1, SLCO4C1) (SLCO5A1) (SLCO6A1)
- (22) organic cation/anion/zwitterion transporter (SLC22A1, SLC22A2, SLC22A3, SLC22A4, SLC22A5, SLC22A6, SLC22A7, SLC22A8, SLC22A9, SLC22A10, SLC22A11, SLC22A12, SLC22A13, SLC22A14, SLC22A15, SLC22A16, SLC22A17, SLC22A18, SLC22A19, SLC22A20)
- (24) Na+/(Ca2+-K+) exchanger (SLC24A1, SLC24A2, SLC24A3, SLC24A4, SLC24A5, SLC24A6)
- (25) mitochondrial carrier (SLC25A1, SLC25A2, SLC25A3, SLC25A4, SLC25A5, SLC25A6, SLC25A7, SLC25A8, SLC25A9, SLC25A10, SLC25A11, SLC25A12, SLC25A13, SLC25A14, SLC25A15, SLC25A16, SLC25A17, SLC25A18, SLC25A19, SLC25A20, SLC25A21, SLC25A22, SLC25A23, SLC25A24, SLC25A25, SLC25A26, SLC25A27, SLC25A28, SLC25A29, SLC25A30, SLC25A31, SLC25A32, SLC25A33, SLC25A34, SLC25A35, SLC25A36, SLC25A37, SLC25A38, SLC25A39, SLC25A40, SLC25A41, SLC25A42, SLC25A43, SLC25A44, SLC25A45, SLC25A46)
SLC31–40 - (32) vesicular inhibitory amino-acid transporter (SLC32A1)
- (33) Acetyl-CoA transporter (SLC33A1)
- (35) nucleoside-sugar transporter (SLC35A1, SLC35A2, SLC35A3, SLC35A4, SLC35A5) (SLC35B1, SLC35B2, SLC35B3, SLC35B4) (SLC35C1, SLC35C2) (SLC35D1, SLC35D2, SLC35D3) (SLC35E1, SLC35E2, SLC35E3, SLC35E4)
- (36) proton-coupled amino-acid transporter (SLC36A1, SLC36A2, SLC36A3, SLC36A4)36A2 ·
- (37) sugar-phosphate/phosphate exchanger (SLC37A1, SLC37A2, SLC37A3, SLC37A4)
- (38) System A & N, sodium-coupled neutral amino-acid transporter (SLC38A1, SLC38A2, SLC38A3, SLC38A4, SLC38A5, SLC38A6, SLC38A10)
- (39) metal ion transporter (SLC39A1, SLC39A2, SLC39A3, SLC39A4, SLC39A5, SLC39A6, SLC39A7, SLC39A8, SLC39A9, SLC39A10, SLC39A11, SLC39A12, SLC39A13, SLC39A14)
- (40) basolateral iron transporter (SLC40A1)
SLC41–48 SLCO1–4 Ion pumps Na+/H+ - Na+/Ca2+ (Na+/(Ca2+-K+)) - Cl-/HCO3- (Band 3) - Cl-formate exchanger - Cl-oxalate exchangersee also solute carrier disorders
B memb: cead, trns (1A, 1C, 1F, 2A, 3A1, 3A2-3, 3D), othrCategories:- Human proteins
- Genes on chromosome 2
- Blood proteins
- Blood antigen systems
- Catalysts
- Clusters of differentiation
- Solute carrier family
- Transfusion medicine
Wikimedia Foundation. 2010.