- ADAM17
-
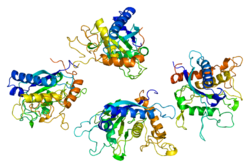
ADAM metallopeptidase domain 17 (ADAM17), also called TACE (tumor necrosis factor-α-converting enzyme), is a 70-kDa enzyme that belongs to the ADAM protein family of disintegrins and metalloproteases.
Contents
Chemical characteristics
ADAM17 is an 824-amino acid polypeptide.[1][2]
Enzymes in this family are transmembrane glycoproteins that are characterized by their conserved, multi-domain structure.
Functions
ADAM17 is understood to be involved in the processing of tumor necrosis factor alpha (TNF-α) at the surface of the cell, and from within the intracellular membranes of the trans-Golgi network. This process, which is also known as 'shedding', involves the cleavage and release of a soluble ectodomain from membrane-bound pro-proteins (such as pro-TNF-α), and is of known physiological importance. ADAM17 was the first 'sheddase' to be identified, and is also understood to play a role in the release of a diverse variety of membrane-anchored cytokines, cell adhesion molecules, receptors, ligands, and enzymes.
Interactions between ADAM17 and pro-TNF-α
Cloning of the TNF-α gene revealed it to encode a 26 kDa type II transmembrane pro-polypeptide that becomes inserted into the cell membrane during its maturation. At the cell surface, pro-TNF-α is biologically active, and is able to induce immune responses via juxtacrine intercellular signaling. However, pro-TNF-α can undergo a proteolytic cleavage at its Ala76-Val77 amide bond, which releases a soluble 17kDa extracellular domain (ectodomain) from the pro-TNF-α molecule. This soluble ectodomain is the cytokine commonly known as TNF-α, which is of pivotal importance in paracrine signaling. This proteolytic liberation of soluble TNF-α is catalyzed by ADAM17.
Interactions between ADAM17 and L-selectin
ADAM17 has a role in the shedding of L-selectin, a cellular adhesion molecule.[3]
Cellular localization of ADAM17
The localization of ADAM17 is speculated to be an important determinant of shedding activity. TNF-α processing has classically been understood to occur in the trans-Golgi network, and be closely connected to transport of soluble TNF-α to the cell surface. However, research that suggests that the majority of mature, endogenous ADAM17 may be localized to a perinuclear compartment, with only a small amount of TACE being present on the cell surface, exists. The localization of mature ADAM17 to a perinulcear compartment, therefore, raises the possibility that ADAM17-mediated ectodomain shedding may also occur in the intracellular environment, in contrast with the conventional model.
ADAM17 as a target for future medicines?
Since TNF-α is a potent and pivotal mediator in the inflammatory process, considerable investment has been made in the research into – and development of – anti-TNF-α agents, for the purpose of reducing the severity of inflammatory responses in disease states (e.g., the medicine etanercept for treatment of rheumatoid arthritis). The inhibition of ADAM17 by a pharmacological agent may represent another means by which the effects of TNF-α can be modulated, although this is yet to be demonstrated clinically in humans.
Functional ADAM17 has been documented to be ubiquitously expressed in the human colon, with increased activity in the colonic mucosa of patients with ulcerative colitis, a main form of inflammatory bowel disease. These data will no doubt contribute to growing interest in the inhibition of ADAM17 as a potential therapeutic strategy.
Other points of interest
Recent in vitro experiments have provided evidence that suggests that ADAM17 may play a prominent role in the Notch signaling pathway, during the proteolytic release of the Notch intracellular domain (from the Notch1 receptor) that occurs following ligand binding. Other experiments have also suggested that expression of ADAM17 may be inhibited by ethanol.[4]
Interactions
ADAM17 has been shown to interact with MAPK1,[5] DLG1[6] and MAD2L1.[7][8]
References
- ^ Black R, Rauch C, Kozlosky C, Peschon J, Slack J, Wolfson M, Castner B, Stocking K, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley K, Gerhart M, Davis R, Fitzner J, Johnson R, Paxton R, March C, Cerretti D (1997). "A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells". Nature 385 (6618): 729–33. doi:10.1038/385729a0. PMID 9034190.
- ^ Moss ML, Jin SL, Milla ME et al. (1997). "Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha". Nature 385 (6618): 733–36. doi:10.1038/385733a0. PMID 9034191.
- ^ Li Y, Brazzell J, Herrera A, Walcheck B (2006). "ADAM17 deficiency by mature neutrophils has differential effects on L-selectin shedding". Blood 108 (7): 2275–9. doi:10.1182/blood-2006-02-005827. PMC 1895557. PMID 16735599. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1895557.
- ^ Taïeb J, Delarche C, Ethuin F, Selloum S, Poynard T, Gougerot-Pocidalo MA, Chollet-Martin S (December 2002). "Ethanol-induced inhibition of cytokine release and protein degranulation in human neutrophils". J. Leukoc. Biol. 72 (6): 1142–7. PMID 12488495.
- ^ Díaz-Rodríguez, Elena; Montero Juan Carlos, Esparís-Ogando Azucena, Yuste Laura, Pandiella Atanasio (Jun. 2002). "Extracellular Signal-regulated Kinase Phosphorylates Tumor Necrosis Factor α-converting Enzyme at Threonine 735: A Potential Role in Regulated Shedding". Mol. Biol. Cell (United States) 13 (6): 2031–44. doi:10.1091/mbc.01-11-0561. ISSN 1059-1524. PMC 117622. PMID 12058067. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=117622.
- ^ Peiretti, Franck; Deprez-Beauclair Paule, Bonardo Bernadette, Aubert Helene, Juhan-Vague Irene, Nalbone Gilles (May. 2003). "Identification of SAP97 as an intracellular binding partner of TACE". J. Cell. Sci. (England) 116 (Pt 10): 1949–57. doi:10.1242/jcs.00415. ISSN 0021-9533. PMID 12668732.
- ^ Nelson, K K; Schlöndorff J, Blobel C P (Nov. 1999). "Evidence for an interaction of the metalloprotease-disintegrin tumour necrosis factor alpha convertase (TACE) with mitotic arrest deficient 2 (MAD2), and of the metalloprotease-disintegrin MDC9 with a novel MAD2-related protein, MAD2beta". Biochem. J. (ENGLAND) 343 Pt 3 (Pt 3): 673–80. ISSN 0264-6021. PMC 1220601. PMID 10527948. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1220601.
- ^ Poghosyan, Zaruhi; Robbins Stephen M, Houslay Miles D, Webster Ailsa, Murphy Gillian, Edwards Dylan R (Feb. 2002). "Phosphorylation-dependent interactions between ADAM15 cytoplasmic domain and Src family protein-tyrosine kinases". J. Biol. Chem. (United States) 277 (7): 4999–5007. doi:10.1074/jbc.M107430200. ISSN 0021-9258. PMID 11741929.
Further reading
- Black RA (2002). "Tumor necrosis factor-alpha converting enzyme". Int. J. Biochem. Cell Biol. 34 (1): 1–5. doi:10.1016/S1357-2725(01)00097-8. PMID 11733179.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Black RA, Rauch CT, Kozlosky CJ et al. (1997). "A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells". Nature 385 (6618): 729–33. doi:10.1038/385729a0. PMID 9034190.
- Moss ML, Jin SL, Milla ME et al. (1997). "Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha". Nature 385 (6618): 733–6. doi:10.1038/385733a0. PMID 9034191.
- Maskos K, Fernandez-Catalan C, Huber R et al. (1998). "Crystal structure of the catalytic domain of human tumor necrosis factor-α-converting enzyme". Proc. Natl. Acad. Sci. U.S.A. 95 (7): 3408–12. doi:10.1073/pnas.95.7.3408. PMC 19849. PMID 9520379. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=19849.
- Patel IR, Attur MG, Patel RN et al. (1998). "TNF-alpha convertase enzyme from human arthritis-affected cartilage: isolation of cDNA by differential display, expression of the active enzyme, and regulation of TNF-alpha". J. Immunol. 160 (9): 4570–9. PMID 9574564.
- Schroeter EH, Kisslinger JA, Kopan R (1998). "Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain". Nature 393 (6683): 382–6. doi:10.1038/30756. PMID 9620803.
- Hirohata S, Seldin MF, Apte SS (1999). "Chromosomal assignment of two ADAM genes, TACE (ADAM17) and MLTNB (ADAM19), to human chromosomes 2 and 5, respectively, and of Mltnb to mouse chromosome 11". Genomics 54 (1): 178–9. doi:10.1006/geno.1998.5544. PMID 9806848.
- Lum L, Wong BR, Josien R et al. (1999). "Evidence for a role of a tumor necrosis factor-alpha (TNF-alpha)-converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dendritic cell survival". J. Biol. Chem. 274 (19): 13613–8. doi:10.1074/jbc.274.19.13613. PMID 10224132.
- Cerretti DP, Poindexter K, Castner BJ et al. (1999). "Characterization of the cDNA and gene for mouse tumour necrosis factor alpha converting enzyme (TACE/ADAM17) and its location to mouse chromosome 12 and human chromosome 2p25". Cytokine 11 (8): 541–51. doi:10.1006/cyto.1998.0466. PMID 10433800.
- Nelson KK, Schlöndorff J, Blobel CP (2000). "Evidence for an interaction of the metalloprotease-disintegrin tumour necrosis factor alpha convertase (TACE) with mitotic arrest deficient 2 (MAD2), and of the metalloprotease-disintegrin MDC9 with a novel MAD2-related protein, MAD2beta". Biochem. J. 343 Pt 3 (Pt 3): 673–80. PMC 1220601. PMID 10527948. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1220601.
- Kärkkäinen I, Rybnikova E, Pelto-Huikko M, Huovila AP (2000). "Metalloprotease-disintegrin (ADAM) genes are widely and differentially expressed in the adult CNS". Mol. Cell. Neurosci. 15 (6): 547–60. doi:10.1006/mcne.2000.0848. PMID 10860581.
- Brou C, Logeat F, Gupta N et al. (2000). "A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE". Mol. Cell 5 (2): 207–16. doi:10.1016/S1097-2765(00)80417-7. PMID 10882063.
- Lee MH, Verma V, Maskos K et al. (2002). "Engineering N-terminal domain of tissue inhibitor of metalloproteinase (TIMP)-3 to be a better inhibitor against tumour necrosis factor-alpha-converting enzyme". Biochem. J. 364 (Pt 1): 227–34. PMC 1222565. PMID 11988096. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1222565.
- Lee MH, Verma V, Maskos K et al. (2002). "The C-terminal domains of TACE weaken the inhibitory action of N-TIMP-3". FEBS Lett. 520 (1–3): 102–6. doi:10.1016/S0014-5793(02)02776-X. PMID 12044879.
- Díaz-Rodríguez E, Montero JC, Esparís-Ogando A et al. (2002). "Extracellular Signal-regulated Kinase Phosphorylates Tumor Necrosis Factor α-converting Enzyme at Threonine 735: A Potential Role in Regulated Shedding". Mol. Biol. Cell 13 (6): 2031–44. doi:10.1091/mbc.01-11-0561. PMC 117622. PMID 12058067. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=117622.
- Mohan MJ, Seaton T, Mitchell J et al. (2002). "The tumor necrosis factor-alpha converting enzyme (TACE): a unique metalloproteinase with highly defined substrate selectivity". Biochemistry 41 (30): 9462–9. doi:10.1021/bi0260132. PMID 12135369.
- Gómez-Gaviro MV, González-Alvaro I, Domínguez-Jiménez C et al. (2002). "Structure-function relationship and role of tumor necrosis factor-alpha-converting enzyme in the down-regulation of L-selectin by non-steroidal anti-inflammatory drugs". J. Biol. Chem. 277 (41): 38212–21. doi:10.1074/jbc.M205142200. PMID 12147693.
- Zheng Y, Schlondorff J, Blobel CP (2003). "Evidence for regulation of the tumor necrosis factor alpha-convertase (TACE) by protein-tyrosine phosphatase PTPH1". J. Biol. Chem. 277 (45): 42463–70. doi:10.1074/jbc.M207459200. PMID 12207026.
PDB gallery 1bkc: CATALYTIC DOMAIN OF TNF-ALPHA CONVERTING ENZYME (TACE)1zxc: Crystal structure of catalytic domain of TNF-alpha converting enzyme (TACE) with inhibitor2a8h: Crystal structure of catalytic domain of TACE with Thiomorpholine Sulfonamide Hydroxamate inhibitor2ddf: Crystal structure of TACE in complex with TAPI-22fv5: Crystal structure of TACE in complex with IK6822fv9: Crystal stucture of TACE in complex with JMV 390-12i47: Crystal structure of catalytic domain of TACE with inhibitorExternal links
1-50 CD1 (a-c, 1A, 1D, 1E) · CD2 · CD3 (γ, δ, ε) · CD4 · CD5 · CD6 · CD7 · CD8 (a) · CD9 · CD10 · CD11 (a, b, c) · CD13 · CD14 · CD15 · CD16 (A, B) · CD18 · CD19 · CD20 · CD21 · CD22 · CD23 · CD24 · CD25 · CD26 · CD27 · CD28 · CD29 · CD30 · CD31 · CD32 (A, B) · CD33 · CD34 · CD35 · CD36 · CD37 · CD38 · CD39 · CD40 · CD41 · CD42 (a, b, c, d) · CD43 · CD44 · CD45 · CD46 · CD47 · CD48 · CD49 (a, b, c, d, e, f) · CD5051-100 CD51 · CD52 · CD53 · CD54 · CD55 · CD56 · CD57 · CD58 · CD59 · CD61 · CD62 (E, L, P) · CD63 · CD64 (A, B, C) · CD66 (a, b, c, d, e, f) · CD68 · CD69 · CD70 · CD71 · CD72 · CD73 · CD74 · CD78 · CD79 (a, b) · CD80 · CD81 · CD82 · CD83 · CD84 · CD85 (a, d, e, h, j, k) · CD86 · CD87 · CD88 · CD89 · CD90 · CD91- CD92 · CD93 · CD94 · CD95 · CD96 · CD97 · CD98 · CD99 · CD100101-150 CD101 · CD102 · CD103 · CD104 · CD105 · CD106 · CD107 (a, b) · CD108 · CD109 · CD110 · CD111 · CD112 · CD113 · CD114 · CD115 · CD116 · CD117 · CD118 · CD119 · CD120 (a, b) · CD121 (a, b) · CD122 · CD123 · CD124 · CD125 · CD126 · CD127 · CD129 · CD130 · CD131 · CD132 · CD133 · CD134 · CD135 · CD136 · CD137 · CD138 · CD140b · CD141 · CD142 · CD143 · CD144 · CD146 · CD147 · CD148 · CD150151-200 CD151 · CD152 · CD153 · CD154 · CD155 · CD156 (a, b, c) · CD157 · CD158 (a, d, e, i, k) · CD159 (a, c) · CD160 · CD161 · CD162 · CD163 · CD164 · CD166 · CD167 (a, b) · CD168 · CD169 · CD170 · CD171 · CD172 (a, b, g) · CD174 · CD177 · CD178 · CD179 (a, b) · CD181 · CD182 · CD183 · CD184 · CD185 · CD186 · CD191 · CD192 · CD193 · CD194 · CD195 · CD196 · CD197 · CDw198 · CDw199 · CD200201-250 CD201 · CD202b · CD204 · CD205 · CD206 · CD207 · CD208 · CD209 · CDw210 (a, b) · CD212 · CD213a (1, 2) · CD217 · CD218 (a, b) · CD220 · CD221 · CD222 · CD223 · CD224 · CD225 · CD226 · CD227 · CD228 · CD229 · CD230 · CD233 · CD234 · CD235 (a, b) · CD236 · CD238 · CD239 · CD240CE · CD240D · CD241 · CD243 · CD244 · CD246 · CD247- CD248 · CD249251-300 CD252 · CD253 · CD254 · CD256 · CD257 · CD258 · CD261 · CD262 · CD264 · CD265 · CD266 · CD267 · CD268 · CD269 · CD271 · CD272 · CD273 · CD274 · CD275 · CD276 · CD278 · CD279 · CD280 · CD281 · CD282 · CD283 · CD284 · CD286 · CD288 · CD289 · CD290 · CD292 · CDw293 · CD294 · CD295 · CD297 · CD298 · CD299301-350 ADAM proteins Matrix metalloproteinases Other Categories:- Human proteins
- Peptidase
- Clusters of differentiation
- Enzymes
- Signal transduction
Wikimedia Foundation. 2010.