- MMP1
-
Interstitial collagenase also known as matrix metalloproteinase-1 (MMP-1) and fibroblast collagenase is an enzyme that in humans is encoded by the MMP1 gene.[1][2][3]
Contents
Function
Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. Most MMP's are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. This gene encodes a secreted enzyme which breaks down the interstitial collagens, types I, II, and III. The gene is part of a cluster of MMP genes which localize to chromosome 11q22.3.[1]
In addition, mechanical force may increase the expression of MMP1 in human periodontal ligament cells.[4]
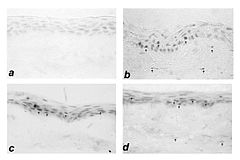 Induction of matrix metalloproteinase 1 in rat corneas by ciprofloxacin, ofloxacin and levofloxacin (b,c,d) compared to artificial tears (a). Reviglio et al., 2003.
Induction of matrix metalloproteinase 1 in rat corneas by ciprofloxacin, ofloxacin and levofloxacin (b,c,d) compared to artificial tears (a). Reviglio et al., 2003.
Interactions
MMP1 has been shown to interact with CD49b.[5][6]
References
- ^ a b "Entrez Gene: MMP1 matrix metallopeptidase 1 (interstitial collagenase)". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=4312.
- ^ Brinckerhoff CE, Ruby PL, Austin SD, Fini ME, White HD (February 1987). "Molecular cloning of human synovial cell collagenase and selection of a single gene from genomic DNA". J. Clin. Invest. 79 (2): 542–6. doi:10.1172/JCI112845. PMC 424122. PMID 3027129. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=424122.
- ^ Pendás AM, Santamaría I, Alvarez MV, Pritchard M, López-Otín C (October 1996). "Fine physical mapping of the human matrix metalloproteinase genes clustered on chromosome 11q22.3". Genomics 37 (2): 266–8. doi:10.1006/geno.1996.0557. PMID 8921407.
- ^ Huang SF, Li YH, Ren YJ, Cao ZG, Long X (August 2008). "The effect of a single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter on force-induced MMP-1 expression in human periodontal ligament cells". Eur. J. Oral Sci. 116 (4): 319–23. doi:10.1111/j.1600-0722.2008.00552.x. PMID 18705799.
- ^ Stricker TP, Dumin JA, Dickeson SK, Chung L, Nagase H, Parks WC, Santoro SA (August 2001). "Structural analysis of the alpha(2) integrin I domain/procollagenase-1 (matrix metalloproteinase-1) interaction". J. Biol. Chem. 276 (31): 29375–81. doi:10.1074/jbc.M102217200. PMID 11359774.
- ^ Dumin JA, Dickeson SK, Stricker TP, Bhattacharyya-Pakrasi M, Roby JD, Santoro SA, Parks WC (August 2001). "Pro-collagenase-1 (matrix metalloproteinase-1) binds the alpha(2)beta(1) integrin upon release from keratinocytes migrating on type I collagen". J. Biol. Chem. 276 (31): 29368–74. doi:10.1074/jbc.M104179200. PMID 11359786.
Further reading
- Krane SM (1995). "Is collagenase (matrix metalloproteinase-1) necessary for bone and other connective tissue remodeling?". Clin. Orthop. Relat. Res. (313): 47–53. PMID 7641497.
- Massova I, Kotra LP, Fridman R, Mobashery S (1998). "Matrix metalloproteinases: structures, evolution, and diversification.". FASEB J. 12 (12): 1075–95. doi:10.1142/S0217984998001256. PMID 9737711.
- Nagase H, Woessner JF (1999). "Matrix metalloproteinases.". J. Biol. Chem. 274 (31): 21491–4. doi:10.1074/jbc.274.31.21491. PMID 10419448.
- Okada Y, Hashimoto G (2002). "[Degradation of extracellular matrix by matrix metalloproteinases and joint destruction]". Seikagaku 73 (11): 1309–21. PMID 11831026.
- Seiki M (2003). "Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion.". Cancer Lett. 194 (1): 1–11. doi:10.1016/S0304-3835(02)00699-7. PMID 12706853.
- Golubkov VS, Strongin AY (2007). "Proteolysis-driven oncogenesis.". Cell Cycle 6 (2): 147–50. PMID 17245132.
External links
PDB gallery 1ayk: INHIBITOR-FREE CATALYTIC FRAGMENT OF HUMAN FIBROBLAST COLLAGENASE, NMR, 30 STRUCTURES1cge: CRYSTAL STRUCTURES OF RECOMBINANT 19-KDA HUMAN FIBROBLAST COLLAGENASE COMPLEXED TO ITSELF1cgf: CRYSTAL STRUCTURES OF RECOMBINANT 19-KDA HUMAN FIBROBLAST COLLAGENASE COMPLEXED TO ITSELF1cgl: STRUCTURE OF THE CATALYTIC DOMAIN OF FIBROBLAST COLLAGENASE COMPLEXED WITH AN INHIBITOR1hfc: 1.56 ANGSTROM STRUCTURE OF MATURE TRUNCATED HUMAN FIBROBLAST COLLAGENASE1su3: X-ray structure of human proMMP-1: New insights into collagenase action2ayk: INHIBITOR-FREE CATALYTIC FRAGMENT OF HUMAN FIBROBLAST COLLAGENASE, NMR, MINIMIZED AVERAGE STRUCTURE2clt: CRYSTAL STRUCTURE OF THE ACTIVE FORM (FULL-LENGTH) OF HUMAN FIBROBLAST COLLAGENASE.2j0t: CRYSTAL STRUCTURE OF THE CATALYTIC DOMAIN OF MMP-1 IN COMPLEX WITH THE INHIBITORY DOMAIN OF TIMP-12tcl: STRUCTURE OF THE CATALYTIC DOMAIN OF HUMAN FIBROBLAST COLLAGENASE COMPLEXED WITH AN INHIBITOR3ayk: CATALYTIC FRAGMENT OF HUMAN FIBROBLAST COLLAGENASE COMPLEXED WITH CGS-27023A, NMR, MINIMIZED AVERAGE STRUCTURE4ayk: CATALYTIC FRAGMENT OF HUMAN FIBROBLAST COLLAGENASE COMPLEXED WITH CGS-27023A, NMR, 30 STRUCTURES966c: CRYSTAL STRUCTURE OF FIBROBLAST COLLAGENASE-1 COMPLEXED TO A DIPHENYL-ETHER SULPHONE BASED HYDROXAMIC ACIDADAM proteins Matrix metalloproteinases Other Categories:- Human proteins
- Peptidase
- Chromosome 11 gene stubs
Wikimedia Foundation. 2010.