- Thermolysin
Thermolysin EC number|3.4.24.27 is a thermostable neutral
metalloproteinase enzyme produced by thegram-positive bacteria "Bacillus thermoproteolyticus". [cite journal | author=Endo, S. | title= Studies on protease produced by thermophilic bacteria | journal= J. Ferment. Technol. | year=1962 | volume=40 | pages=346-353] It requires one zinc ion for enzyme activity and four calcium ions ions for structural stability. [cite journal | author=Tajima M, Urabe I. "et al". | title= Role of calcium ions in the thermostability of thermolysin and Bacillus subtilis var. amylosacchariticus neutral protease | journal= Eur. J. Biochem. | year=1976 | volume=64 | issue=1 | pages=243-247 | pmid=819262] Thermolysin specifically catalyzes thehydrolysis ofpeptide bond s containing hydrophobic amino acids. However thermolysin is also widely used for peptide bond formation through the reverse reaction of hydrolysis. [cite journal | author=Trusek-Holownia A. | title= Synthesis of ZAlaPheOMe, the precursor of bitter dipeptide in the two-phase ethyl acetate-water system catalysed by thermolysin | journal= J. Biotechnol. | year=2003 | volume=102 | issue=2 | pages=153-163 | pmid=12697393] Thermolysin is the most stable member of a family of metalloproteinases produced by various "Bacillus " species. These enzymes are also termed 'neutral' proteinases or thermolysin -like proteinases (TLPs).ynthesis
Like all bacterial
extracellular proteases thermolysin is first synthesised by the bactrium as a pre-proenzyme. [cite journal | author=Yasukawa K, Kusano M, Inouye K. | title= A new method for the extracellular production of recombinant thermolysin by co-expressing the mature sequence and pro-sequence in Escherichia coli | journal= Protein Eng. Des. Sel. | year=2007 | volume=20 | issue=8 | pages=375-383| pmid=17616558] Thermolysin is synthesized as a pre-proenzyme consisting of asignal peptide 28 amino acids long, a pro-peptide 204 amino acids long and the mature enzyme itself 316 amino acids in length. The signal peptide acts as a signal fortranslocation of pre-prothermolysin to the bacterial cytoplasmic membrane. In theperiplasm pre-prothermolysin is then processed into prothermolysin by asignal peptidase . The prosequence then acts as amolecular chaperone and leads to autocleavage of the peptide bond linking pro and mature sequences. The mature protein is then secreted into the extracellular medium. [cite journal | author=Inouye K, Kusano M. "et al". | title=Engineering, expression, purification, and production of recombinant thermolysin | journal=Biotechnol. Annu. Rev. | year=2007 | volume=13 | pages=43-64 | pmid=17875473]tructure
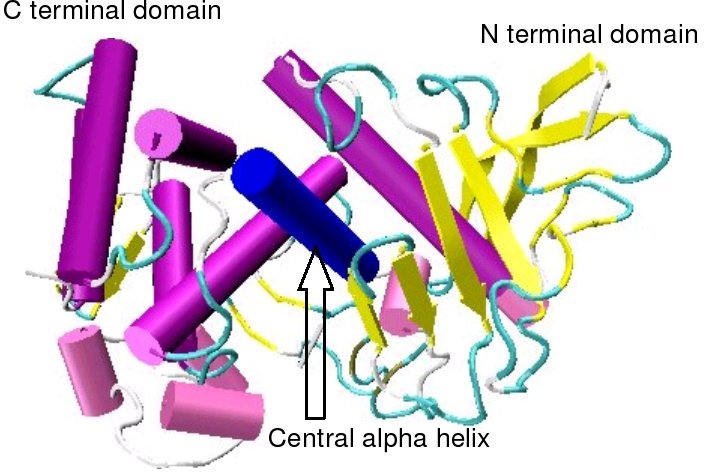
left|thumb|300px|right|Structure_of_thermolysin_taken_from_pdb file [http://www.pdb.org/pdb/explore.do?structureId=3TMN 3TMN] ]
Thermolysin consists of abeta pleated sheet richN-terminal domain and aalpha helical richC-terminal domain. These two domains are connected by a central alpha helix.References
External links
*
Wikimedia Foundation. 2010.