- Beta-secretase 1
-
Beta-secretase 1 (BACE1) also known as beta-site APP cleaving enzyme 1 (beta-site amyloid precursor protein cleaving enzyme 1), memapsin-2 (membrane-associated aspartic protease 2), and aspartyl protease 2 (ASP2) is an enzyme that in humans is encoded by the BACE1 gene.[1]
β-Secretase is an aspartic-acid protease important in the pathogenesis of Alzheimer's disease, and in the formation of myelin sheaths in peripheral nerve cells.[2] The transmembrane protein contains two active site aspartate residues in its extracellular protein domain and may function as a dimer.
Contents
Role in Alzheimer's disease
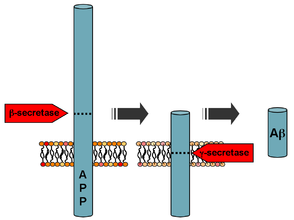
Generation of the 40 or 42 amino acid-long amyloid-β peptides that aggregate in the brain of Alzheimer's patients requires two sequential cleavages of the amyloid precursor protein (APP). Extracellular cleavage of APP by BACE creates a soluble extracellular fragment and a cell membrane-bound fragment referred to as C99. Cleavage of C99 within its transmembrane domain by γ-secretase releases the intracellular domain of APP and produces amyloid-β. Since alpha-secretase cleaves APP closer to the cell membrane than BACE does, it removes a fragment of the amyloid-β peptide. Initial cleavage of APP by alpha-secretase rather than BACE prevents eventual generation of amyloid-β.
Unlike APP and the presenilin proteins important in γ-secretase, no known mutations in the gene encoding BACE cause early-onset, familial Alzheimer's disease, which is a rare form of the disorder. However, levels of this enzyme have been shown to be elevated in the far more common late-onset sporadic Alzheimer's. The physiological purpose of BACE's cleavage of APP and other transmembrane proteins is unknown. BACE2 is a close homolog of BACE1 with no reported APP cleavage in vivo.
BACE inhibitors
Drugs to block this enzyme (BACE inhibitors) in theory would prevent the build up of beta-amyloid and may help slow or stop the disease. Several companies are in the early stages of development and testing of this new potential class of treatment.[3][4]
Relationship to plasmepsin
Beta secretase, a vertebrate (human) aspartic-acid protease, is distantly related to the pathogenic aspartic-acid protease plasmepsin, which is a potential target for future anti-malarial drugs.[5]
See also
- Beta-secretase 2
References
- ^ Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M (October 1999). "Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE". Science 286 (5440): 735–41. doi:10.1126/science.286.5440.735. PMID 10531052.
- ^ Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C (October 2006). "Control of peripheral nerve myelination by the beta-secretase BACE1". Science 314 (5799): 664–6. doi:10.1126/science.1132341. PMID 16990514. Lay summary – The Scientist.
- ^ Walker LC, Rosen RF (July 2006). "Alzheimer therapeutics-what after the cholinesterase inhibitors?". Age Ageing 35 (4): 332–5. doi:10.1093/ageing/afl009. PMID 16644763.
- ^ Baxter EW, Conway KA, Kennis L, Bischoff F, Mercken MH, Winter HL, Reynolds CH, Tongue BA, Luo C, Scott MK, Huang Y, Braeken M, Pieters SM, Berthelot DJ, Masure S, Bruinzeel WD, Jordan AD, Parker MH, Boyd RE, Qu J, Alexander RS, Brenneman DE, Reitz AB (September 2007). "2-Amino-3,4-dihydroquinazolines as inhibitors of BACE-1 (beta-site APP cleaving enzyme): Use of structure based design to convert a micromolar hit into a nanomolar lead". J. Med. Chem. 50 (18): 4261–4. doi:10.1021/jm0705408. PMID 17685503.
- ^ Russo I, Babbitt S, Muralidharan V, Butler T, Oksman A, Goldberg DE (February 2010). "Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte". Nature 463 (7281): 632–6. doi:10.1038/nature08726. PMC 2826791. PMID 20130644. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2826791. l laysource = sciencedaily.com Lay summary.
External links
Further reading
- Hong L, He X, Huang X et al. (2005). "Structural features of human memapsin 2 (beta-secretase) and their biological and pathological implications". Acta Biochim. Biophys. Sin. (Shanghai) 36 (12): 787–92. doi:10.1093/abbs/36.12.787. PMID 15592644.
- Johnston JA, Liu WW, Todd SA et al. (2006). "Expression and activity of beta-site amyloid precursor protein cleaving enzyme in Alzheimer's disease". Biochem. Soc. Trans. 33 (Pt 5): 1096–100. doi:10.1042/BST20051096. PMID 16246054.
- Dominguez DI, Hartmann D, De Strooper B (2006). "BACE1 and presenilin: two unusual aspartyl proteases involved in Alzheimer's disease". Neuro-degenerative diseases 1 (4–5): 168–74. doi:10.1159/000080982. PMID 16908986.
- Zacchetti D, Chieregatti E, Bettegazzi B et al. (2007). "BACE1 expression and activity: relevance in Alzheimer's disease". Neuro-degenerative diseases 4 (2–3): 117–26. doi:10.1159/000101836. PMID 17596706.
PDB gallery 1fkn: Structure of Beta-Secretase Complexed with Inhibitor1m4h: Crystal Structure of Beta-secretase complexed with Inhibitor OM00-31sgz: Crystal Structure of Unbound Beta-Secretase Catalytic Domain.1tqf: Crystal structure of human Beta secretase complexed with inhibitor1w50: APO STRUCTURE OF BACE (BETA SECRETASE)1w51: BACE (BETA SECRETASE) IN COMPLEX WITH A NANOMOLAR NON-PEPTIDIC INHIBITOR1xn2: New substrate binding pockets for beta-secretase.1xn3: Crystal structure of Beta-secretase bound to a long inhibitor with additional upstream residues.1xs7: Crystal Structure of a cycloamide-urethane-derived novel inhibitor bound to human brain memapsin 2 (beta-secretase).1ym2: Crystal structure of human beta secretase complexed with NVP-AUR2001ym4: Crystal structure of human beta secretase complexed with NVP-AMK6402b8l: Crystal structure of human beta secretase complexed with inhibitor2b8v: Crystal structure of human Beta-secretase complexed with L-L000430,4692f3e: Crystal Structure of the Bace complex with AXQ093, a macrocyclic inhibitor2f3f: Crystal Structure of the Bace complex with BDF488, a macrocyclic inhibitor2fdp: Crystal structure of beta-secretase complexed with an amino-ethylene inhibitor2g94: Crystal structure of beta-secretase bound to a potent and highly selective inhibitor.2hiz: Crystal Structure of human beta-secretase (BACE) in the presence of an inhibitor2hm1: Crystal Structure of human beta-secretase (BACE) in the presence of an inhibitor (2)2iqg: Crystal Structure of Hydroxyethyl Secondary Amine-based Peptidomimetic Inhibitor of Human Beta-Secretase (BACE)2irz: Crystal structure of human Beta-secretase complexed with inhibitor2is0: Crystal structure of human Beta-secretase complexed with inhibitor2of0: X-ray crystal structure of beta secretase complexed with compound 52ohk: X-ray crystal structure of beta secretase complexed with 1-amino-isoquinoline2ohl: X-ray crystal structure of beta secretase complexed with 2-aminoquinoline2ohm: X-ray crystal structure of beta secretase complexed with N~3~-benzylpyridine-2,3-diamine2ohn: X-ray crystal structure of beta secretase complexed with 4-(4-fluorobenzyl)piperidine2ohp: X-ray crystal structure of beta secretase complexed with compound 32ohq: X-ray crystal structure of beta secretase complexed with compound 42ohr: X-ray crystal structure of beta secretase complexed with compound 6a2ohs: X-ray crystal structure of beta secretase complexed with compound 6b2oht: X-ray crystal structure of beta secretase complexed with compound 72ohu: X-ray crystal structure of beta secretase complexed with compound 8b2ph6: Crystal Structure of Human Beta Secretase Complexed with inhibitorHydrolase: proteases (EC 3.4) 3.4.11-19: Exopeptidase Dipeptidyl peptidase (Cathepsin C, Dipeptidyl peptidase-4) · Tripeptidyl peptidase (Tripeptidyl peptidase I, Tripeptidyl peptidase II)Other/ungrouped3.4.21-24: Endopeptidase Serine proteases · Cysteine protease · Aspartic acid protease · Metalloendopeptidases
Other/ungrouped: Amyloid precursor protein secretase (Alpha secretase, Beta-secretase 1, Beta-secretase 2, Gamma secretase)3.4.99: Unknown Vertebrate Pathogenic Plant Cathepsin D · ECategories:- Human proteins
- Neurobiology
- Enzymes
- Integral membrane proteins
- EC 3.4.23
- Alzheimer's disease
Wikimedia Foundation. 2010.