- Miraculin
-
Miraculin 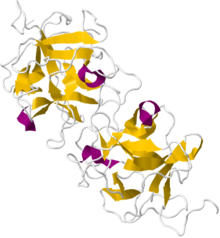
Crystallographic structure of a dimeric miraculin like protein from seeds of Murraya koenigii.[1] Identifiers Symbol MIRA_RICDU UniProt P13087 Other data Miraculin is a natural sugar substitute, a glycoprotein extracted from the fruit of Synsepalum dulcificum.[2] The berry, which contains active polyphenols, was first documented by explorer Chevalier des Marchais, who searched for many different fruits during a 1725 excursion to its native West Africa.
Miraculin itself is not sweet. However, after the taste buds are exposed to miraculin, ordinarily sour foods, such as citrus, are perceived as sweet. This effect lasts up to an hour.
Miraculin works by binding to the sweet receptors on the tongue. Miraculin's effect lasts as long as the protein is bound to the tongue, which can be up to an hour. It makes most acidic foods taste sweet, but does not improve the taste of bitter things.[3]
The active substance, isolated by Prof. Kenzo Kurihara (栗原 堅三 Kurihara Kenzō),[4] a Japanese scientist, was named miraculin after the miracle fruit when Kurihara published his work in Science in 1968.[5]
Contents
Glycoprotein structure
Miraculin was first sequenced in 1989 and was found to be a glycoprotein consisting of 191 amino acids and some carbohydrate chains.[6]
Miraculin occurs as a tetramer (98.4 kDa), a combination of 4 monomers group by dimer. Within each dimer 2 miraculin glycoproteins are linked by a disulfide bridge.[7]
SIGNAL (29) MKELTMLSLS FFFVSALLAA AANPLLSAA 1-50 DSAPNPVLDI DGEKLRTGTN YYIVPVLRDH GGGLTVSATT PNGTFVCPPR 51-100 VVQTRKEVDH DRPLAFFPEN PKEDVVRVST DLNINFSAFM PNPGPETISS 101-150 WCRWTSSTVW RLDKYDESTG QYFVTIGGVK FKIEEFCGSG FYKLVFCPTV 151-191 CGSCKVKCGD VGIYIDQKGR GRRLALSDKP FAFEFNKTVY F Amino acids sequence of glycoprotein miraculin unit adapted from Swiss-Prot biological database of protein sequences.[8] The molecular weight of the glycoprotein is 24.6 kDa including 3.4 kDa (13.9% of the weight) of sugar constituted (on molar ratio) of glucosamine (31%), mannose (30%), fucose (22%), xylose (10%) and galactose (7%).[2]
The taste modifying protein, miraculin, has seven cystein residues in a molecule composed of 191 amino acid residues. Both tetramer miraculin and native dimer miraculin in its crude state have the taste-modifying activity of turning sour tastes into sweet tastes.[9]
Sweetness properties
Miraculin, unlike curculin (another taste-modifying agent), is not sweet by itself, but it can change a sour beverage into a sweet beverage, even for a long period after consumption. The anti-sweet compound, Gymnemic acid suppresses the sweet taste of miraculin, like it does for sucrose. [7] The duration and intensity of the taste-modifying phenomena depends on various factors — miraculin concentration, duration of contact of the miraculin with the tongue, and acid concentration. Maximum sweet-induced response has been shown to be equivalent to the sweetness of 17% sucrose solution.
Glycoprotein is sensitive to heat: when heated over 100 °C, miraculin loses its taste-modifying property. Miraculin activity is inactivated at pH below 3 and pH above 12 at room temperature. The sweet modifying effect stays at pH 4 (in acetate buffer), for 6 months at 5 °C.[2]
The detailed mechanism of the taste-inducing behaviour is still unknown. It has been suggested that the miraculin protein can change the structure of taste cells on the tongue.[10] As a result, the sweet receptors are activated by acids, which are sour in general. This effect remains until the taste buds return to normal. The two histidine residues (i.e. His29 and His59) appear to be mainly responsible for the taste-modifiying behavior.[11] One site maintains the attachment of the protein to the membranes while the other (with attached xylose or arabinose) activates the sweet receptor membrane in acid solutions.[7] Further research at the University of Tokyo using a system of cultured cells which allowed the testing of human taste receptors at various pH to uncover the mechanism. As already known miraculin binds strongly to the sweet taste receptors on our tongues, however it does not activate receptors at neutral pH. Once acid is introduced, the miraculin protein changes shape in such a way that it turns on the sweet receptors it is bound to, causing an ultra-sweet sensation without affecting other flavours tasted. Once the acidic food is swallowed, miraculin returns to the inactive shape until the next acidic food comes along. This can continue for about an hour while it is still bound to the taste receptor. http://news.discovery.com/human/miracle-fruit-berry-everything-taste-sweet-110926.html
As a sweetener
As miraculin is a readily soluble protein and relatively heat stable, it is a potential sweetener in acidic food (e.g. soft drinks). Japanese researchers' more or less successful attempts to mass produce it are focused on recombinant technology. While attempts to express it in yeast and tobacco plants have failed, researchers have succeeded in preparing genetically modified E. coli bacteria,[12] lettuce[10][13] and tomatoes[14] that express miraculin. The scientists' crops resulted in 40 micrograms of miraculin per gram of lettuce leaves, which was considered a large amount.[10] Two grams of lettuce leaves produced roughly the same amount of miraculin as in one miracle fruit berry.[15]
Miraculin was never approved for use as a sweetener by the United States Food and Drug Administration (FDA). In the 1970s the Miralin Company planned on bringing miraculin to market and was founded with investments by Reynolds Metals, Barclays, and Prudential. Legal advice and contact with the FDA indicated that miraculin would be approved as generally recognized as safe as the berries had been eaten for centuries in Africa with no reports of adverse reactions (substances used in food prior to January 1, 1958, through either scientific procedures or through experience based on common use in food can be designated GRAS). However, on the eve of the product's launch in 1974 the FDA determined that miraculin would be considered a food additive and thus require years more testing. At this point the company's investment capital could not sustain them and Miralin folded. Afterward, Miralin requested the FDA documents under the freedom of information act. The documents were nearly completely blacked out, and the rationale for the sudden change in regulation was not revealed.[16][17][18]
Miraculin has a novel food status in the European Union.[citation needed] However it is approved in Japan as a harmless additive, according to the List of Existing Food Additives published by the Ministry of Health and Welfare (published by JETRO).
Limitations
Miraculin is a non-heat-stable protein, subject to denaturation from heating, and thus miracle berries are not taste-bud active when cooked.[10]
While miraculin changes the perception of taste, it does not change the food's chemistry, leaving the mouth and stomach vulnerable to the high acidity of some foods, such as lemon juice, that may cause irritation if eaten in large quantities.[10]
See also
References
- ^ PDB 3IIR; Gahloth D, Selvakumar P, Shee C, Kumar P, Sharma AK (February 2010). "Cloning, sequence analysis and crystal structure determination of a miraculin-like protein from Murraya koenigii". Arch. Biochem. Biophys. 494 (1): 15–22. doi:10.1016/j.abb.2009.11.008. PMID 19914199.
- ^ a b c Theerasilp S, Kurihara Y (August 1988). "Complete purification and characterization of the taste-modifying protein, miraculin, from miracle fruit". J. Biol. Chem. 263 (23): 11536–9. PMID 3403544. http://www.jbc.org/cgi/reprint/263/23/11536.
- ^ Aaron Rowe (2006), Super Lettuce Turns Sour Sweet, http://www.wired.com/science/discoveries/news/2006/12/72251
- ^ Ui, Michio (1999). "「薬学会賞受賞栗原堅三氏の業績 : それは「奇蹟の果物」からはじまった [Kurihara Kenzou Performance Award Pharmaceutical Society said: it is a "miracle fruit"]" (in Japanese). Farumashia (The Pharmaceutical Society of Japan) 35 (4): 365. http://ci.nii.ac.jp/naid/110003660406/en/.
- ^ Kurihara K, Beidler LM (September 1968). "Taste-modifying protein from miracle fruit". Science 161 (3847): 1241–3. doi:10.1126/science.161.3847.1241. PMID 5673432.
- ^ Theerasilp S, Hitotsuya H, Nakajo S, Nakaya K, Nakamura Y, Kurihara Y (April 1989). "Complete amino acid sequence and structure characterization of the taste-modifying protein, miraculin". J. Biol. Chem. 264 (12): 6655–9. PMID 2708331. http://www.jbc.org/cgi/reprint/264/12/6655.
- ^ a b c Kurihara Y (1992). "Characteristics of antisweet substances, sweet proteins, and sweetness-inducing proteins". Crit Rev Food Sci Nutr 32 (3): 231–52. doi:10.1080/10408399209527598. PMID 1418601.
- ^ UniProtKB/Swiss-Prot database entry P13087
- ^ Igeta H, Tamura Y, Nakaya K, Nakamura Y, Kurihara Y (September 1991). "Determination of disulfide array and subunit structure of taste-modifying protein, miraculin". Biochim. Biophys. Acta 1079 (3): 303–7. doi:10.1016/0167-4838(91)90073-9. PMID 1911854.
- ^ a b c d e Rowe, Aaron (2006-12-07). "Super Lettuce Turns Sour Sweet". Wired Magazine. http://www.wired.com/science/discoveries/news/2006/12/72251. Retrieved 2008-07-22. ""Sweet receptors sit on taste buds and wait for sweet molecules to come along and set them off," explained Göran Hellekant, a miraculin researcher and professor of physiology and pharmacology at the University of Minnesota. "Normally, they can only be set off by chemicals that are legitimately sweet, but miraculin may distort their shape a bit so that they become responsive to acids, instead of sugar and other sweet things.""
- ^ Ito K, Asakura T, Morita Y, Nakajima K, Koizumi A, Shimizu-Ibuka A, Masuda K, Ishiguro M, Terada T, Maruyama J, Kitamoto K, Misaka T, Abe K (August 2007). "Microbial production of sensory-active miraculin". Biochem. Biophys. Res. Commun. 360 (2): 407–11. doi:10.1016/j.bbrc.2007.06.064. PMID 17592723.
- ^ Matsuyama T, Satoh M, Nakata R, Aoyama T, Inoue H (April 2009). "Functional expression of miraculin, a taste-modifying protein in Escherichia coli". J. Biochem. 145 (4): 445–50. doi:10.1093/jb/mvn184. PMID 19122203.
- ^ Sun HJ, Cui ML, Ma B, Ezura H (January 2006). "Functional expression of the taste-modifying protein, miraculin, in transgenic lettuce". FEBS Lett. 580 (2): 620–6. doi:10.1016/j.febslet.2005.12.080. PMID 16406368.
- ^ Kazuhisa Kato, Riichiro Yoshida, Ayako Kikuzaki, Tadayoshi Hirai, Hirofumi Kuroda, Kyoko Hiwasa-Tanase, Kenichi Takane, Hiroshi Ezura, Tsuyoshi Mizoguchi (August 2010). "Molecular Breeding of Tomato Lines for Mass Production of Miraculin in a Plant Factory". J. Agric. Food Chem. 58 (17): 9505–10. doi:10.1021/jf101874b. PMID 20695489. http://pubs.acs.org/doi/abs/10.1021/jf101874b.
- ^ Slater, Joanna (2007-03-30). "To Make Lemons Into Lemonade, Try 'Miracle Fruit'". Wall Street Journal. http://online.wsj.com/article_email/SB117522147769754148-lMyQjAxMDE3NzM1MDIzMjAxWj.html. Retrieved 2008-05-28. "[...]two grams produce roughly the same effect as one miracle fruit."
- ^ [1] How Stuff Works, Flavor Tripping article. retrieved 2009-11-10
- ^ BBC News Online. retrieved 2009-11-10
- ^ Wall Street Journal. 2007-03-30. Retrieved 2009-11-10
External links
Categories:- Proteins
- Taste-modifying
- Sweeteners
- Food science
Wikimedia Foundation. 2010.
