- Polyphenol
-
 Plant-derived polyphenol, tannic acid, formed by esterification of ten equivalents of the phenylpropanoid-derived gallic acid to a monosaccharide (glucose) core from primary metabolism.
Plant-derived polyphenol, tannic acid, formed by esterification of ten equivalents of the phenylpropanoid-derived gallic acid to a monosaccharide (glucose) core from primary metabolism.
Polyphenols[1][2] (noun, pronunciation of the singular /pɑli'finəl/[3] or /pɑli'fɛnəl/) are a structural class of natural, synthetic, and semisynthetic organic chemicals characterized by the presence of large multiples of phenol structural units (right). The number and characteristics of these phenol structures underlie the unique physical, chemical, and biological (metabolic, toxic, therapeutic, etc.) properties of particular members of the polyphenol class. The name derives from poly-, from the ancient Greek word πολύς (polus, meaning “many, much”) and the word phenol which refers to a chemical structure formed by attaching to an aromatic benzenoid (phenyl) ring, an hydroxyl (-OH) group akin to that found in alcohols (hence the "-ol" suffix). The term polyphenol appears to have been in use since 1894.[3]
Definition of the term polyphenol
Basis in the organic chemical and natural products literature
The most research-informed and chemistry-aware definition of the polyphenol term describes the class as (i) generally moderately water-soluble compounds, (ii) with molecular weight of 500–4000 Da, (iii) >12 phenolic hydroxyl groups, and (iv) 5–7 aromatic rings per 1000 Da, where the limits to these ranges are necessarily somewhat flexible.[4][1]
Examples of the compound class include the black tea antioxidant theaflavin-3-gallate shown below, and the hydrolyzable tannin, tannic acid, shown above. Notably, the historically important chemical class of the tannins is a subset of the polyphenols. These examples highlight the high density of phenolic substructures that characterize the class and underlie their properties, and introduces their origin as plant-derived substances (phytochemicals).[1]
Individual polyphenols engage in reactions related to their core structure—standard phenolic reactions (e.g., ionization, oxidations to ortho- and para-quinones, and other underlying aromatic transformations related to the presence of the phenolic hydroxyl, etc.; see phenol image above)—as well as reactions related to their peripheral structures (e.g., nucleophilic additions, oxidative and hydrolytic bond cleavages, etc.).[5] As critically, per the definition, the polyphenols display behaviors more explicitly limited to the polyphenol class—for instance, formation of particular metal complexes (e.g., intense blue-black iron(III) complexes), and precipitation of proteins and particular amine-containing organics (e.g., particular alkaloid natural products).[4]
Underlying importance of class definition to structure-activity understanding
Less than rigorous use of the polyphenol term toward the lower molecular weight end of the range is understandable; it is associated, for instance, with the limited understanding of chemical structure in play when a plant extract is first studied (see, for instance, pharmacognosy to understand the process from plant extract to determined chemical structures), and with a desire to associate newly discovered phenolic substances with particularly well-studied, pharmacologically interesting "true" polyphenols (e.g., for pattern, see [6],[7]).
The need to distinguish between structure classes, even in rough terms, relates to the critical concept of structure-activity relationships (SAR)—the fact that the biological activities of chemical agents are structure-derived and structure-dependent.[8],[9] Hence, understanding of the way in which phenolic chemical structures vary (disambiguating types) is important to understanding of how and why biological activities vary with those structures (see, e.g., [10],[1]). This in turn underlies good correlative research and consumer decision-making about use of any phenolic agent, including polyphenols.[7]
Catalog of conflicting definitions based on common and web-usage
In the Merriam Webster[3] and Babylon on-line[11] dictionaries, rather than differentiation based on the presence of many (poly) phenolic substructures, polyphenols are instead defined as molecules with more than one hydroxyl group on one or more phenol unit per molecule. At wiktionary, they are alcohols [sic] containing two or more benzene rings that each have at least one hydroxyl group attached,[12] a definition that likewise confounds simple natural phenols and polyphenols, and further confounds the very different properties of alcoholic and phenolic hydroxyl groups. Likewise, the term polyphenol is increasingly confounded in some parts of the bioactivity and analytical research literature with simple and mid-molecular weight plant phenolic natural products (see next paragraph), though less so in literatures of modern organic and natural products chemistry where more precise understandings of naming apply.[13]
In the parts of literature where polyphenol is used less rigorously, the term is used almost interchangeably with other terms/structures such as simple natural phenols, intermediate weight phenolics, and the newer and less precedented term phenoloids.[14]
Finally, some web sources give definitions based solely on antioxidant properties or other health benefits, or on plant origins.[15][16][17]The former is rejected as a sufficient condition and an oversimplification (not all antioxidants are polyphenols and vice versa, and phenolic benefits and toxicity are defined by their SAR). The latter is rejected on chemical terms, because identical chemical agents can be isolated from various natural sources other than plants,[18][19] or with effort can be prepared synthetically.[20][21]
Different aims of lexicography vs. chemical nomenclature in polyphenol definition
It is generally understood that the aims of normal English lexicography versus chemical nomenclature vary and are to an extent at odds. Dictionaries of English words on the web or otherwise collect and report the meanings of words as their uses appear and change over time. Chemical nomenclature on the other hand (with IUPAC nomenclature as the best example) is necessarily more restrictive: it aims to standardize communication and practice so that when a chemical term is used, it has a fixed meaning relating to chemical structure, and thereby, can give insights into chemical properties and derived molecular functions. These differing aims can have profound effects on valid understanding in chemistry, especially with regard to chemical classes made popular for perceived health benefits; this is particularly true in the new media age of the web, where word meanings can rapidly change.
The tension between nomenclature vs. other definitions is evident in the polyphenol literature, and correlates with commercial interest in promoting nutraceutical and supplement products (e.g., [22]), and with broad scientific interest in new phenolic substances[23]—where new discoveries must be tied to earlier ones even though new reports often precede structure assignment; hence, first assignments of activity with structure (SAR) may be incomplete or inaccurate.[24][25][26]
While the polyphenol definition provides only rough boundaries, they are structurally and functionally relevant and useful; despite web definitions to the contrary, all chemicals presenting a phenolic group are not polyphenols, and so cannot be expected to provide the health benefits associated with, for instance, tea and wine polyphenols (see below). Moreover, phenol itself, C6H5OH, the simplest phenol, along with other examples, are not therapeutic agents; phenol itself is caustic, and toxic if taken internally. As structural complexity increases from phenol to di-,tri- and oligophenols, and on through to commercially and medically important true polyphenols, one encounters hepatotoxins, drugs, endocrine disruptors—the problematic bisphenol A can fit some loose polyphenol definitions—industrial dyes, plant pigments (i.e., all manner of bioactive agents).
In short, the general and specific biologic and therapeutic properties of all phenolic substances connect to chemical structure, and chemical definitions and classifications such as "polyphenol" must substitute in literature and common use for more detailed structural knowledge. The ability to connect beneficial properties—value in commercial tanning, anti-proliferative pharmacology, etc.—with the polyphenol chemical class will only succeed with meaningful nomenclature applied to this and other phenol classes.
Origin of the rigorous polyphenol definition
Building off of earlier natural products research efforts of E.C. Bate-Smith, A. Swain and T. White that characterized specific structural characteristics common to plant phenolics used in tanning, research chemist Edwin Haslam and colleagues developed the four-point definition based on solubility, molecular weight, numbers of phenolic substructures, and aromatic ring density presented above. This definition is now know as the White–Bate-Smith–Swain–Haslam (WBSSH) definition.[4] It is the definition applied in the highest level research and practical discussions of the structure class.[1]
Disambiguation of polyphenols from simpler and polymeric phenols
 Plant-derived theaflavin-3-gallate, formed by esterification of two equivalents of gallic acid to a theaflavin core. this example meets several of the WBSSH definition points (see below), but not the phenol-count criterion.
Plant-derived theaflavin-3-gallate, formed by esterification of two equivalents of gallic acid to a theaflavin core. this example meets several of the WBSSH definition points (see below), but not the phenol-count criterion.
 Ellagic acid, a dimer of gallic acid, and a core-type component of polyphenols
Ellagic acid, a dimer of gallic acid, and a core-type component of polyphenols
Notably, the four-point definition disambiguates in rough terms the higher molecular weight and more structurally and functionally complex polyphenols from plant-derived "simple" natural phenols—monoaromatics such gallic and caffeic acids—and from other plant-originating dimer and trimer types of phenolic natural product classes—e.g., lignans and flavanoids—that occupy the interesting structure and property "space" between simple and polyphenols. For instance, the gallic acid dimer, ellagic acid (M.W. 302, right) is an example of a dimeric phenolic compound at the core of various natural products. An example raspberry ellagitannin (M.W. ~2450),[27]—a possible radical-scavenging antioxidant—on the other hand, is a polyphenol per se, containing 6 ellagic acid-type components and two additional monomeric phenolics, for a total of 14 gallic acid units (and all of their substituent phenolic hydroxyl groups). Hence, there are structural, property-associated, and functional characteristics which underlie the naming differences between complex polyphenols and their component phenolic substructures, just as the complex antibiotic structure, vancomycin, and any other complex organic chemical structure is clearly disambiguated from its component parts (e.g., in the vancomycin case, from glucosyl-vancosamine, hydroxychlorotyrosine, etc.) in order to make sense in communicating.
The association of the polyphenol term with a component class, the physiologically and pharmacologically interesting flavanoids[28]—with 2 core aromatic rings, and commonly with 4-6 core phenolic hydroxyl groups (0-9 possible)—is an example of nomenclature and structure-function confounding. Simple flavanoids have chemical behaviors and biologic toxicities and therapeutic potentials distinct from the polyphenols; e.g., flavonoids display a range of solubilities depending on substitution pattern, and typically stabilize protein structures in aqueous solution rather than instigating precipitation (c.f. [29][30] and defined polyphenol solubility and protein precipitation properties, above). Physical, chemical, and biologic properties (SAR) associated with phenolic structural classes cannot be generalized across the broad expanses between classes, and sometimes not much beyond a particular individual chemical structure.
Polyphenols, versus phenolic polymers
The use of the term polyphenol does not indicate that this class of larger phenols are a type of polymer. Strictly speaking, polymers require the presence of repeating monomeric structures, either ordered or random, and this requirement is generally unmet in polyphenols. This is true, even though in some molecules such as tannic acid—shown above—and the condensed tannins, particular phenolic units appear more than once in the structure. An example which highlights the distinction and the dependence of properties on structure (i.e., SAR) is the relation of polyphenols to the true phenolic polymer lignin, a complex, random polymer that constitutes the woody tissue of gymnosperms. Lignin is the most abundant phenol-containing natural substance and, in general, the most abundant terrestrial polymeric substance. Lignin is formed by extensive radical-mediated cross-linking of simple phenols derived from plant phenylpropanoid biosynthetic pathways. It is very high molecular weight, very highly oxidized, and truly polymeric—it is wood, insoluble, and without strong ongoing therapeutic interest. It is not a polyphenol, akin to the ellagitannin—pictured and discussed above—that meets WBSSH definition components (moderate water-solubility, phenolic count, etc.)—which is not fully oxidized, and is of significant therapeutic interest.
Note, there is theoretically no reason to exclude the possibility of limited polymerization of a phenolic monomer, such that it would result in formation of a soluble structure that would be both a polyphenol by the WBSSH definition, and have the repeating characteristics of a true polymer. The arecatannin-type natural products from Ceylonese cassia bark and Areca seed may be considered as an example of such.[31]
Chemical Structure and Properties
Structural features
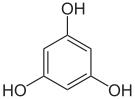
In common with simple and mid-molecular weight phenolic dimers and trimers (natural phenols), the phenol substructures of polyphenols have various further nomenclatures depending on the number of phenolic hydroxyl groups. A phenol, per se, is the term for a substructure with one phenolic hydroxyl group, catechol- and resorcinol-types (benzenediols) have two, and pyrogallol- and phloroglucinol-types (benzenetriols) have three. Polyphenols always have heteroatom substituents other than hydroxyl groups; as might be expected, ether and ester linkages are common, as are various carboxylic acid derivatives (see theaflavin gallate image).




Phenol Pyrocatechol Pyrogallol Resorcinol Phloroglucinol  The biphenyl/biaryl substructure of polyphenols, here as prepared by synthetic chemists using the copper-mediated Ullmann reaction. This substructure can be observed in the structure of ellagic acid above. The carbon-carbon bond in biaryls in nature is also synthesized though a metal-mediated coupling reaction, often involving iron.
The biphenyl/biaryl substructure of polyphenols, here as prepared by synthetic chemists using the copper-mediated Ullmann reaction. This substructure can be observed in the structure of ellagic acid above. The carbon-carbon bond in biaryls in nature is also synthesized though a metal-mediated coupling reaction, often involving iron.
 A C-glucoside substructure in the phenol-saccharide conjugate structure of puerarin, a mid-molecular weight plant natural product (though not a polyphenol)—where the attachment of the phenol to the saccharide is via a carbon-carbon bond, as in polyphenols with this substructure. Also a part of the structure is an isoflavone, which contains the 10-atom benzopyran "fused ring" system seen in polyphenols.
A C-glucoside substructure in the phenol-saccharide conjugate structure of puerarin, a mid-molecular weight plant natural product (though not a polyphenol)—where the attachment of the phenol to the saccharide is via a carbon-carbon bond, as in polyphenols with this substructure. Also a part of the structure is an isoflavone, which contains the 10-atom benzopyran "fused ring" system seen in polyphenols.
 An example of the spiro-type substructure found in polyphenols—where two rings are joined at a single shared point—with illustration of the two stereoisomers that can arise because of such junctures, labeled R and S (from the CIP system, for rectus/right/clockwise and sinister/left/counterclockwise, respectively).
An example of the spiro-type substructure found in polyphenols—where two rings are joined at a single shared point—with illustration of the two stereoisomers that can arise because of such junctures, labeled R and S (from the CIP system, for rectus/right/clockwise and sinister/left/counterclockwise, respectively).
As the earlier images suggest, polyphenol compositions are normally limited to carbon, hydrogen and oxygen in undefined proportion. Carbon frameworks can be complex, arising from various biosynthetic pathways aimed at plant and related secondary metabolites; e.g., the 7-atom ring (7-membered ring) appearing in theaflavin structure above is an example of a "carbocycle" that is of a nonbenzenoid aromatic tropolone type. In addition, various biaryls and triaryls occur (e.g., biphenyls), see figure at right, and benzopyrans and normal and C-glucoside derivatives frequently appear (further figure at right) —e.g. in condensed, complex and hydrolyzable tannins such as in stenophyllanin A (1), acutissimin B (2), mongolicain A (3), stenophynin A (4), mongolicanin (5), and mongolicin B (6). Spiro-type structures as illustrated at right appear as in preceding compound (3); furanoid, pyrone, and other heterocycles appear as in compounds (4) and (6); (diaryl)methyl structures as in (1), (2), and (6); as do pyrans and dioxins, etc.[31] Because of the preponderance of saccharide-derived core structures (e.g., see tannic acid image above), as well as spiro- and other structure types, natural chiral (stereo) centers abound.
Chemical properties
Polyphenols are molecules producing autofluorescence, especially lignin and the phenolic part of suberin.
Polyphenols are reactive species toward oxidation.[32] The Fenton's reagent, a mixture producing reactive oxygen species, used in association with a photo-oxidation system may be used to treat oil mill waste water.[33] A complex mixture of polyphenols, found in food for example, can undergo autoxidation during the ageing process. Simple natural phenols can lead to the formation of B-type procyanidins in wines[34] or in model solutions.[35][36] This is correlated to the non enzymatic browning color change characteristic of this process.[37] This phenomenon can be observed in foods like carrot purees.[38] ABTS may be used to characterise polyphenol oxidation products.[39]
Polyphenols can interact with proteins (case of tannins) and other food matrices.
Chemical synthesis
Polyphenols can be synthetised chemically from phenol to produce a mixture of phenylene and oxyphenylene.[40]
Natural phenols can be enzymatically polymerised. Laccase and peroxidase induced the polymerization of syringic acid to give a poly(1,4-phenylene oxide) bearing a carboxylic acid at one end and a phenolic hydroxyl group at the other.[41]
Classification and nomenclature
In terms of chemical classification, polyphenols are generally divided into hydrolyzable tannins (gallic acid esters of glucose and other sugars or cyclitols) and phenylpropanoids, such as lignins, flavonoids, and condensed tannins. This division is derived from the variety of simple polyphenolic units derived from secondary plant metabolism as well as classical divisions based upon the relative importance of each base component to different fields of study. Tannin chemistry originated in the importance of tannic acid to the tanning industry; lignins to the chemistry of soil and plant structure; and flavonoids to the chemistry of plant secondary metabolites for plant defense, and flower color (e.g. from anthocyanins).
Chemical uses
Some polyphenols are traditionally used as dyes. For instance, in the Indian subcontinent, the pomegranate peel, high in tannins and other polyphenols, or its juice, is employed in the dyeing of non-synthetic fabrics.[42]
Polyphenols, especially, tannins, can be used as precursors in green chemistry[43] notably to produce plastics or resins by polymerisation with[44] or without the use of formaldehyde[45] or adhesives for particleboards.[46] The aims are generally to make use of plant residues from grape, olive (called pomaces) or pecan shells left after processing.
A special form of polyphenols-derived resins are the EUV resists.[47]
Polyphenols are also used for the production of creosote to treat wood.
Biology
Occurrence in nature
The most abundant polyphenols are the condensed tannins, found in virtually all families of plants, and comprising up to 50% of the dry weight of leaves. Plant polyphenols have antioxidant action and may help reduce tooth decay.[48]
Some polyphenols produced by plants in case of pathogens attacks are called phytoalexins. Such compounds can be implied in the hypersensitive response of plants. High levels of polyphenols in some woods can explain their natural preservation against rot.[49]
Cucumbers grown on vermicompost may be less susceptible to striped cucumber beetle attack due to a higher induced level of polyphenols in the plant.
Polyphenols can be involved in allelopathic interactions in soil[50] or in water.[51] The fruit pulp of the plant Liriope muscari contains phenolic compounds which inhibit its own seeds germination.
Polyphenols can be a source of pollution in sites near processing plants producing olive oil, coffee (see coffee wastewater) or paper. Laccases (found for instance in the fungal species Panellus stipticus) can be used in bioremediation.
Polyphenol content can be an element of chemotaxonomy.[52]
Polyphenols can also be found in animals. In arthropods like insects[53] and crustaceans[54] polyphenols play a role in epicuticle hardening (sclerotization). The hardening of the cuticle is due to the presence of a polyphenol oxidase.[55] In crustaceans, there is a second oxidase activity leading to cuticle pigmentation.[56] There is apparently no polyphenol tanning occurring in arachnids cuticle.[57]
Metabolism
Biosynthesis
Polyphenol oxidase (PPO) is an enzyme that catalyses the oxidation of o-diphenols to produce o-quinones. It is the rapid polymerisation of o-quinones to produce black, brown or red polyphenolic pigments that is the cause of fruit browning. In insects, PPO serves for the cuticle hardening.[58]
Laccase is a major enzyme that initiates the cleavage of hydrocarbon rings, which catalyzes the addition of a hydroxyl group to phenolic compounds. This enzyme can be found in fungi like Panellus stipticus, organisms able to break down lignin, a complex aromatic polymer in wood that is highly resistant to degradation by conventional enzyme systems.
Anthracyclines or hypericin[59] are derived from polyketides cyclisation.[60]
The glycosylated form increases the solubility of polyphenols.[61]
Content in food
See also: List of phytochemicals in food A koala eating Eucalyptus leaves. These contain formylated phloroglucinol derivatives, a type of polyphenol.[62]
A koala eating Eucalyptus leaves. These contain formylated phloroglucinol derivatives, a type of polyphenol.[62]
Polyphenols are considered antinutrients, compounds that interfere with the absorption of nutrients as a mechanism of plant defense against herbivores. Leaf protein concentrates used as animal food can also be rich in polyphenols.[63] Animals produce salivary proteins, like protein IB5, as herbivore adaptations to plant defense, to counter the tannins astringent effect. Some animals like the greater glider or the koala can digest low nutrient foliage, specifically eucalypt leaf matter, which contains a variety of phenolic and terpenoid compounds and a high concentration of lignified fiber. Some frugivores like birds are reported to not sense astringency.
In human food
Main articles: Natural phenols and polyphenols in wine and Natural phenols and polyphenols in teaSee also: List of phytochemicals in foodPolyphenols from algae (phlorotannins) may be used as additives to prevent lipid oxidation during fish preservation.[64]
Role of processing in phenolic composition
Phenolic and carotenoid compounds with antioxidant properties in vegetables have been found to be retained significantly better through steaming than through frying.[65]
Polyphenols in wine, beer and various nonalcoholic juice beverages can be removed using finings, substances that are usually added at or near the completion of the processing of brewing.
Marketing argument
The presence of polyphenols is a marketing argument to sell functional foods, dietary supplements or anti-aging creams.
Functional foods may contain polyphenols. For superfruit beverages, which may include extracts from fruits like açai or pomegranate, the detailed composition of polyphenols is usually not revealed on the nutrition label. Instead, there may be an ORAC value given for the in vitro antioxidant capacity of the product. Polyphenol-enriched drinks may actually deliver the intended blend of bioavailable polyphenols, which would normally require consumption of several different plant-derived foods.[66]
As a matter of pharmacovigilance, health benefits from using these products have not been scientifically confirmed or approved by regulatory authorities and may only be supported by preliminary research. Accordingly, there are no recommended Dietary Reference Intake levels established for polyphenols as exist for essential nutrients.
Potential health effects
Main article: Health effects of polyphenolsResearch on polyphenols
Bioavailability
Questions on the relationship between health benefits and polyphenols generally revolve around bioavailability.[67]
Compared with the effects of polyphenols in vitro, the effects in vivo, although the subject of ongoing research, are limited and vague. The reasons for this are 1) the absence of validated in vivo biomarkers, especially for inflammation or carcinogenesis; 2) long-term studies failing to demonstrate effects with a mechanism of action, specificity or efficacy; and 3) invalid applications of high, unphysiological test concentrations in the in vitro studies, which are subsequently irrelevant for the design of in vivo experiments.[68] In rats, polyphenols absorbed in the small intestine[69] may be bound in protein-polyphenol complexes modified by intestinal microflora enzymes,[70] allowing derivative compounds formed by ring-fission to be better absorbed.[71][72]
Matrix effect
The poor bioavailability of polyphenols in vivo may be due to a matrix effect. Casein found in milk added to a polyphenol-rich food like tea may reduce the absorbed polyphenols content[73] by the formation of a complex as demonstrated in vitro.[74][75] Moreover other substances such as caffeine can form insoluble complexes with polyphenols.[76]
Polyphenols may also interact with fibers like pectins and have a positive effect in large intestine accessibility.[77]
Analysis
The analysis techniques are those of phytochemistry: extraction, isolation, structural elucidation,[78] then quantification.
Extraction
Extraction of polyphenols[79] can be performed using a solvent like water, hot water, methanol, methanol/formic acid, methanol/water/acetic or formic acid etc. Liquid liquid extraction can be also performed or countercurrent chromatography. Solid phase extraction can also be made on C18 sorbent cartridges. Other techniques are ultrasonic extraction, heat reflux extraction, microwave-assisted extraction,[80] critical carbon dioxide,[81] pressurized liquid extraction[82] or use of ethanol in an immersion extractor.[83] The extraction conditions (temperature, extraction time, ratio of solvent to raw material, solvent and concentrations) have to be optimized.
Mainly found in the fruit skins and seeds, high levels of polyphenols may reflect only the measured extractable polyphenol (EPP) content of a fruit which may also contain non-extractable polyphenols.[84]
Concentration can be made by ultrafiltration.[85] Purification can be achieved by preparative chromatography.
Analysis techniques
Reversed-phase HPLC plot of separation of phenolic compounds. Smaller natural phenols formed individual peaks while tannins form a hump.
Phosphomolybdic acid is used as a reagent for staining phenolics in thin layer chromatography. Polyphenols can be studied by the mean of spectroscopy, especially in the ultra violet domain, by fractionation or paper chromatography. They can also be analysed by chemical characterisation.
Instrumental chemistry analyses include separation by high performance liquid chromatography (HPLC), and especially by reversed-phase liquid chromatography (RPLC), can be coupled to mass spectrometry. Purified compounds can be identified by the mean of nuclear magnetic resonance.
Microscopy analysis
The DMACA reagent is an histological dye specific to polyphenols used in microscopy analyses. The autofluorescence of polyphenols can also be used, especially for localisation of lignin and suberin.
Quantification
A method for polyphenolic content quantification is volumetric titration. An oxidizing agent, permanganate, is used to oxidize known concentrations of a standard tannin solution, producing a standard curve. The tannin content of the unknown is then expressed as equivalents of the appropriate hydrolyzable or condensed tannin.[86]
Some methods for quantification of total polyphenol content are based on colorimetric measurements. Some tests are relatively specific to polyphenols (for instance the Porter's assay). Total phenols (or antioxidant effect) can be measured using the Folin-Ciocalteu reaction. Results are typically expressed as gallic acid equivalents. Polyphenols are seldom evaluated by antibodies technologies.[87]
Other tests measure the antioxidant capacity of a fraction. Some make use of the ABTS radical cation which is reactive towards most antioxidants including phenolics, thiols and vitamin C.[88] During this reaction, the blue ABTS radical cation is converted back to its colorless neutral form. The reaction may be monitored spectrophotometrically. This assay is often referred to as the Trolox equivalent antioxidant capacity (TEAC) assay. The reactivity of the various antioxidants tested are compared to that of Trolox, which is a vitamin E analog.
Other antioxidant capacity assays which use Trolox as a standard include the diphenylpicrylhydrazyl (DPPH), oxygen radical absorbance capacity (ORAC),[89] ferric reducing ability of plasma (FRAP)[90] assays or inhibition of copper-catalyzed in vitro human low-density lipoprotein oxidation.[91]
New methods including the use of biosensors can help monitor the content of polyphenols in food.[92]
Quantitation results produced by the mean of diode array detector-coupled HPLC are generally given as relative rather than absolute values as there is a lack of commercially available standards for every polyphenolic molecules.
Other techniques
Chemometrics analyses on acquired data can be performed to compare samples from different origins.
Genetic analysis
In Vitis vinifera cells suspensions, polyphenol production is regulated by sugars levels.[93]
See also
References
- ^ a b c d e S. Quideau, D. Deffieux, C. Douat-Casassus, & L. Pouységu, 2011. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl. 2011, 50(3):586–621; doi:10.1002/anie.201000044
- ^ Stéphane Quideau, 2011. "Why bother with Polyphenols," Groupe Polyphenols website, www.groupepolyphenols.com/index.php?option=com_content&view=article&id=53&Itemid=59&b528026c36a38313c3bc0e90a25fbe0c=7012a845601d61b99d4b8fbc24b709de, accessed 15 February 2011.
- ^ a b c Polyphenol on www.merriam-webster.com online dictionary
- ^ a b c Plant polyphenols (vegetable tannins): gallic acid metabolism. Haslam E. and Cai Y., Nat Prod Rep, 1994, 11, pp. 41–66, doi:10.1039/NP9941100041
- ^ J.W. Drynan, M.N. Clifford, J. Obuchowicz & N. Kuhnert, The chemistry of low molecular weight black tea polyphenols, Nat. Prod. Rep., 2010, 27, 417-462
- ^ www.sciencewatch.com/dr/tt/2011/11-febtt-AGR/, accessed 30 August 2011.
- ^ a b www.naturalproductsinsider.com/articles/2008/08/polyphenols-and-flavonoids-in-product-formulation.aspx
- ^ http://www.britannica.com/EBchecked/topic/1357082/pharmaceutical-industry/260313/Strategies-for-drug-design-and-production?anchor=ref925327, accessed 30 August 2011.
- ^ www.ilsi.org/ResearchFoundation/Pages/DevelopmentalToxicityDatabase.aspx, accessed 30 August 2011.
- ^ G. Cao, E. Sofic & R.L. Prior, 1997, Antioxidant and prooxidant behavior of flavanoids: Structure-activity relationships, Free Radical Biology & Medicine, 1997, 22(5), 749–760.
- ^ Polyphenol on dictionary.babylon.com on line dictionary
- ^ Polyphenol definition on www.thefreedictionary.com online dictionary
- ^ Plant polyphenols: chemical properties, biological activities, and synthesis. Quideau S, Deffieux D, Douat-Casassus C and Pouységu L, Chromatographia, Volume 60, Supplement 1, S93-S100, Angew Chem Int Ed Engl. 2011, 50(3):586–621; doi:10.1002/anie.201000044
- ^ I. Papp, P. Apati, V. Andrasek, A. Blazovics, A. Balazs, L. Kursinszki, G. C. Kite, P. J. Houghton & A. Kery, 2004, LC-MS analysis of antioxidant plant phenoloids, Chromatographia, 60(Suppl.), S93-S100
- ^ www.wellsphere.com/arthritis-article/polyphenols/1334347, accessed 2 September 2011.
- ^ www.medterms.com/script/main/art.asp?articlekey=16619, Polyphenol, on www.medterms.com on line dictionary, accessed 2 September 2011.
- ^ www.cancer.gov/dictionary/?print=1&cdrid=256573, Polyphenol, www.cancer.gov/dictionary, accessed 2 September 2011.
- ^ K. L. Van Alstyne á J. J. McCarthy III C. L. Hustead á D. O. Duggins, 1999, Geographic variation in polyphenolic levels of Northeastern Pacific kelps and rockweeds, Marine Biology (1999) 133:371-379; via the web at <faculty.wwu.edu/kathyva/MB99.pdf>, accessed 2 Sept. 2011.
- ^ P.D. Steinberg, J.A. Estes & F.C. Winter, 1995, Evolutionary consequences of food chain length in kelp forest communities, Proc. Natl. Acad. Sci. USA 92: 8145-8148.
- ^ S. Quideau, 2011, Organic chemistry: Triumph for unnatural synthesis, Nature 474, 459–460 (23 June 2011) doi:10.1038/474459a
- ^ S.A. Snyder, A. Gollner & M.I. Chiriac, 2011, Regioselective reactions for programmable resveratrol oligomer synthesis, Nature 474, 461–466 (23 June 2011) doi:10.1038/nature10197
- ^ www.naturalproductsinsider.com/articles/2008/08/polyphenols-and-flavonoids-in-product-formulation.aspx, accessed 30 August 2011.
- ^ www.sciencewatch.com/dr/tt/2011/11-febtt-AGR, accessed 30 August 2011.
- ^ For instance, when the term polyphenol appeared in the Medical Subject Headings (MeSH) database of the National Library of Medicine in relation to a 1980 report on sclerotization induced by extracts from the Surinam cockroach—see next two reference—the NLM entry's "note" on use of the term polyphenol states: "GEN only; [One should] use the precise structure header, most commonly in the FLAVONOIDS group; this [polyphenol] term only refers vaguely to phenolic (aromatic) hydroxyls…".
- ^ MeSH Polyphenols
- ^ Mosconi Bernardini, P; Cersosimo, A (1979). "Histochemical and biophysical study of cuticle sclerotization in Pycnoscelus surinamensis L. (Blattaria)". Basic and applied histochemistry 23 (3): 203–10. PMID 533516.
- ^ Cardiovascular disease and phytochemicals. Anonymous. C. Hamilton et al.
- ^ FlavonoidViewer.jp
- ^ B. A. Bohm., 1998, Introduction to flavonoids (Amsterdam: Harwood Academic), pp. 175-178.
- ^ L. Chebil, C. Humeau, J. Anthoni, F. Dehez, J.-M. Engasser & M. Ghoul, 2007, Solubility of flavonoids in organic solvents, J. Chem. Eng. Data, 52(5), 1552–1556. DOI: 10.1021/je7001094.
- ^ a b Isolation and structure elucidation of tannins. G. Nonaka, Pure & Appl. Chem.,Vol. 61, No. 3, pp. 357–360, 1989.
- ^ Estimating the selectivity of ozone in the removal of polyphenols from vinasse. Santos M.A, Bonilla Venceslada J.L, Martin Martin A and Garcia Garcia I, Journal of chemical technology and biotechnology, 2005, vol. 80, no4, pp. 433–438
- ^ Photo-Fenton Oxidation of Oil Mill Waste Water. Chemical degradation and biodegradability increase. Pietro Canepa, Fabio Cauglia, Paolo Caviglia and Elisabetta Chelossi, Environmental Science And Pollution Research, Volume 10, Number 4, 217–220, doi:10.1065/espr2001.12.104.7
- ^ Tandem mass spectrometry of the B-type procyanidins in wine and B-type dehydrodicatechins in an autoxidation mixture of (+)-catechin and (-)-epicatechin. Weixing Sun, Miller Jack M., Journal of mass spectrometry, 2003, vol. 38, no4, pp. 438–446
- ^ Identification of autoxidation oligomers of flavan-3-ols in model solutions by HPLC-MS/MS. Fei He, Qiu-Hong Pan, Ying Shi, Xue-Ting Zhang and Chang-Qing Duan, Journal of Mass Spectrometry, Volume 44 Issue 5, Pages 633 – 640, 2008, doi:10.1002/jms.1536
- ^ Nonenzymic autoxidative phenolic browning reactions in a caffeic acid model system. Johannes J. L. Cilliers and Vernon L. Singleton, J. Agric. Food Chem., 1989, 37 (4), pp 890–896, doi:10.1021/jf00088a013
- ^ Nonenzymic Autoxidative Reactions of Caffeic Acid in Wine. Johannes J. L. Cilliers 1 and Vernon L. Singleton, Am. J. Enol. Vitic. 41:1:84-86, 1990.
- ^ Phenolic Autoxidation Is Responsible for Color Degradation in Processed Carrot Puree. Talcott S. T. and Howard L. R., J. Agric. Food Chem., 1999, 47 (5), pp 2109–2115, doi:10.1021/jf981134n
- ^ ABTS radical-driven oxidation of polyphenols: Isolation and structural elucidation of covalent adducts. A.M. Osman, K.K.Y. Wong and A. Fernyhough, Biochemical and Biophysical Research Communications, Volume 346, Issue 1, 21 July 2006, Pages 321–329, doi:10.1016/j.bbrc.2006.05.118
- ^ Oguchi, Takahisa; Tawaki, Shin-Ichiro; Uyama, Hiroshi; Kobayashi, Shiro (1999). "Soluble polyphenol". Macromolecular Rapid Communications 20 (7): 401–3. doi:10.1002/(SICI)1521-3927(19990701)20:7<401::AID-MARC401>3.0.CO;2-6.
- ^ Enzymatic Polymerization of Natural Phenol Derivatives and Enzymatic Synthesis of Polyesters from Vinyl Esters. Hiroshi Uyama, Ryohei Ikeda, Shigeru Yaguchi and Shiro Kobayashi, from book Polymers from Renewable Resources, Chapter 9, pp 113–127, ACS Symposium Series, Vol. 764, doi:10.1021/bk-2000-0764.ch009
- ^ K. K. Jindal, R. C. Sharma (2004). Recent trends in horticulture in the Himalayas. Indus Publishing. ISBN 8173871620. http://books.google.com/?id=LlogqveEFVgC. "... bark of tree and rind of fruit is commonly used in ayurveda ... also used for dyeing ..."
- ^ Polshettiwar, Vivek; Varma, Rajender S. (2008). "Greener and expeditious synthesis of bioactive heterocycles using microwave irradiation". Pure and Applied Chemistry 80 (4): 777–90. doi:10.1351/pac200880040777.
- ^ Reaction of polyphenols with formaldehyde. W. E. Hillis, Gerda Urbach, Journal of Applied Chemistry, Volume 9, Issue 12, pages 665–673, December 1959, doi:10.1002/jctb.5010091207
- ^ Fukuoka, Tokuma; Uyama, Hiroshi; Kobayashi, Shiro (2003). "Synthesis of Ultrahigh Molecular Weight Polyphenols by Oxidative Coupling". Macromolecules 36 (22): 8213–5. doi:10.1021/ma034803t.
- ^ Pizzi, A.; Valenezuela, J.; Westermeyer, C. (1994). "Low formaldehyde emission, fast pressing, pine and pecan tannin adhesives for exterior particleboard". Holz als Roh- und Werkstoff 52 (5): 311–5. doi:10.1007/BF02621421.
- ^ “Development of EUV Resists Based on Non-polymeric Macromolecules with polymeric Macromolecules with Polyphenol Derivatives. Masatoshi Echigo, Dai Oguro, Takeo Watanabe, Hiroo Kinoshita, Yukiko Kikuchi and Iwao Nishiyama, Poster, 2006 International Symposium on Extreme Ultraviolet Lithography 15–18 October 2006 Barcelona, Spain
- ^ Ferrazzano GF, Amato I, Ingenito A, Zarrelli A, Pinto G, Pollio A.,"Plant polyphenols and their anti-cariogenic properties: a review." Molecules. 2011;16(2):1486-507
- ^ Hart, John H.; Hillis, W. E. (1974). "Inhibition of wood-rotting fungi by stilbenes and other polyphenols in Eucalyptus sideroxylon". Phytopathology 64 (7): 939–48. doi:10.1094/Phyto-64-939.
- ^ Popa, V; Dumitru, M; Volf, I; Anghel, N (2008). "Lignin and polyphenols as allelochemicals". Industrial Crops and Products 27 (2): 144–9. doi:10.1016/j.indcrop.2007.07.019.
- ^ Nakai, S (2000). "Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa". Water Research 34 (11): 3026–32. doi:10.1016/S0043-1354(00)00039-7.
- ^ Li, Li; Li, Min-Hui; Xu, Li-Jia; Guo, Na; Wu-Lan, Ta-Na; Shi, Ren-Bing; Peng, Yong; Xiao, Pei-Gen (June 2010). "Distribution of seven polyphenols in several medicinal plants of Boraginaceae in China". Journal of Medicinal Plants Research 4 (12): 1216–21. http://www.academicjournals.org/jmpr/PDF/pdf2010/18June/Li%20et%20al.pdf.
- ^ The source of lipids and polyphenols for the insect cuticle: The role of fat body, oenocytes and oenocytoids. V.B. Wigglesworth, Tissue and Cell, Volume 20, Issue 6, 1988, Pages 919–932, doi:10.1016/0040-8166(88)90033-X
- ^ The Occurrence and Significance of Phenolic Hardening in the Newly Formed Cuticle of Crustacea decapoda. R. Dennell, Proc. R. Soc. Lond. B 30 September 1947 vol. 134 no. 877 485–503, doi:10.1098/rspb.1947.0027
- ^ The distribution of phenoloxidases and polyphenols during cuticle formation. M. Locke and N. Krishnan, Tissue and Cell, Volume 3, Issue 1, 1971, Pages 103–126, doi:10.1016/S0040-8166(71)80034-4
- ^ Phenolic Tanning and Pigmentation of the Cuticle in Carcinus maenas. G. Krishnan, Quarterly Journal of Microscopical Science, Vol s3-92, 333–342
- ^ The Epicuticle of an Arachnid, Palamneus swammerdami. G. Krishnan, Quarterly Journal of Microscopical Science, Vol s3-95, 371–381
- ^ Polyphenols and their quinone derivatives in the cuticle of the desert locust, Schistocerca gregaria (Forskål). S.R.A. Maleka, Comparative Biochemistry and Physiology, Volume 2, Issue 1, January 1961, Pages 35–50, doi:10.1016/0010-406X(61)90071-8
- ^ A Review of the Hypothetical Biogenesis and Regulation of Hypericin synthesis via the Polyketide Pathway in Hypericum perforatum and Experimental Methods Proposed to Evaluate the Hypothesis. Loren W. Walker, Portland State University , May, 1999
- ^ Polyketide Biosynthesis. Christian Hertweck, Angew. Chem. Int. Ed. 2009, 48, 4688–4716, doi:10.1002/anie.200806121
- ^ Polyphenol Glucosylating Activity in Cell Suspensions of Grape (Vitis vinifera). Mark N. Krasnow and Terence M. Murphy, J. Agric. Food Chem., 2004, 52 (11), pp 3467–3472, doi:10.1021/jf035234r
- ^ Distribution of foliar formylated phloroglucinol derivatives amongst Eucalyptus species. B. M. Eschler, D. M. Pass, R. Willis and W. J. Foley, Biochemical Systematics and Ecology, Volume 28, Issue 9, November 2000, Pages 813–824, doi:10.1016/S0305-1978(99)00123-4
- ^ Rambourg, J. C.; Monties, B. (1983). "Determination of polyphenolic compounds in leaf protein concentrates of lucerne and their effect on the nutritional value". Qualitas Plantarum Plant Foods for Human Nutrition 33 (2–3): 169–72. doi:10.1007/BF01091304.
- ^ Enhancing the quality of seafood products through new preservation techniques and seaweed-based antioxidants. Thesis, Tao Wang, 2009
- ^ Miglio C, Chiavaro E, Visconti A, Fogliano V, Pellegrini N (2008). "Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables". J Agric Food Chem 56 (1): 139–47. doi:10.1021/jf072304b. PMID 18069785.
- ^ Bioavailability of multiple components following acute ingestion of a polyphenol-rich juice drink. Gina Borges, William Mullen, Adam Mullan, Michael E. J. Lean, Susan A. Roberts and Alan Crozier, Molecular Nutrition & Food Research, Special Issue: New Perspectives on Dietary Polyphenols, Volume 54, Issue Supplement 2, pages S268–S277, July 2010, doi:10.1002/mnfr.200900611
- ^ Bioavailability of the Polyphenols: Status and Controversies. Massimo D’Archivio, Carmelina Filesi, Rosaria Varì, Beatrice Scazzocchio and Roberta Masella, Int J Mol Sci. 2010; 11(4): 1321–1342, doi:10.3390/ijms11041321, PMC PMC2871118
- ^ Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 81:(1) 243S-5S, 2005. Dietary polyphenols and health: Proceedings of the 1st International Conference on Polyphenols and Health
- ^ Evaluation of polyphenol bioavailability in rat small intestine. Carbonaro M, Grant G and Pusztai A, Eur J Nutr. 2001 Apr;40(2):84–90, PubMed
- ^ Protein-polyphenol interactions and in vivo digestibility of buckwheat groat proteins. Skrabanja V, Laerke HN and Kreft I, Pflugers Arch. 2000;440 (5 Suppl):R129-31, PubMed
- ^ Polyphenols and health: What compounds are involved? D. Del Rio, L.G. Costa, M.E.J. Lean and A. Crozier, Nutrition, Metabolism & Cardiovascular Diseases, Volume 20, Issue 1, Pages 1–6 (January 2010), doi:10.1016/j.numecd.2009.05.015
- ^ Intake and bioaccessibility of total polyphenols in a whole diet. Fulgencio Saura-Calixto, José Serrano and Isabel Goñi, Food Chemistry, Volume 101, Issue 2, 2007, Pages 492–501, doi:10.1016/j.foodchem.2006.02.006
- ^ Addition of milk prevents vascular protective effects of tea. Mario Lorenz, Nicoline Jochmann, Amélie von Krosigk, Peter Martus, Gert Baumann, Karl Stangl and Verena Stangl, Eur Heart J (2007) 28 (2): 219–223, doi:10.1093/eurheartj/ehl442
- ^ Polyphenol−β-Casein Complexes at the Air/Water Interface and in Solution: Effects of Polyphenol Structure. V. Aguié-Béghin, P. Sausse, E. Meudec, V. Cheynier and R. Douillard, J. Agric. Food Chem., 2008, 56 (20), pp 9600–9611, doi:10.1021/jf801672x
- ^ An Investigation of the interactions between milk proteins and tea polyphenols. P.J. Brown and W.B. Wright, Journal of Chromatography A, Volume 11, 1963, Pages 504–514, doi:10.1016/S0021-9673(01)80953-5
- ^ Tea: the Plant and its Manufacture; Chemistry and Consumption of the Beverage. Balentine D. A., Harbowy M. E. and Graham H. N., Prog Clin Biol Res. 1984;158:29–74, PubMed
- ^ Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. Aprikian O, Duclos V, Guyot S, Besson C, Manach C, Bernalier A, Morand C, Rémésy C and Demigné C, J Nutr. 2003 Jun;133(6):1860-5, PubMed
- ^ Isolation and structure elucidation of the major individual polyphenols in carob fibre. R. W. Owen, R. Haubner, W. E. Hull, G. Erben, B. Spiegelhalder, H. Bartsch and B. Haber, Food and Chemical Toxicology, Volume 41, Issue 12, December 2003, Pages 1727–1738, doi:10.1016/S0278-6915(03)00200-X
- ^ Polyphenol extraction from foods. Maria teresa Escribano-Bailon and Celestino Santos-Buelga
- ^ Pan, X (2003). "Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves". Chemical Engineering and Processing 42 (2): 129–33. doi:10.1016/S0255-2701(02)00037-5.
- ^ Palma, M; Taylor, L (1999). "Extraction of polyphenolic compounds from grape seeds with near critical carbon dioxide". Journal of Chromatography A 849 (1): 117–24. doi:10.1016/S0021-9673(99)00569-5. PMID 10444839.
- ^ Alonsosalces, R; Korta, E; Barranco, A; Berrueta, L; Gallo, B; Vicente, F (2001). "Pressurized liquid extraction for the determination of polyphenols in apple". Journal of Chromatography A 933 (1–2): 37–43. doi:10.1016/S0021-9673(01)01212-2. PMID 11758745.
- ^ Sineiro, J.; Domínguez, H.; Núñez, M. J.; Lema, J. M. (1996). "Ethanol extraction of polyphenols in an immersion extractor. Effect of pulsing flow". Journal of the American Oil Chemists' Society 73 (9): 1121–5. doi:10.1007/BF02523372.
- ^ Arranz, Sara; Saura-Calixto, Fulgencio; Shaha, Shika; Kroon, Paul A. (2009). "High Contents of Nonextractable Polyphenols in Fruits Suggest That Polyphenol Contents of Plant Foods Have Been Underestimated". Journal of Agricultural and Food Chemistry 57 (16): 7298–303. doi:10.1021/jf9016652. PMID 19637929.
- ^ Nawaz, H; Shi, J; Mittal, G; Kakuda, Y (2006). "Extraction of polyphenols from grape seeds and concentration by ultrafiltration". Separation and Purification Technology 48 (2): 176–81. doi:10.1016/j.seppur.2005.07.006.
- ^ Tannin-measuring techniques. Alice S. Tempel, Journal of Chemical Ecology, Volume 8, Number 10, 1289–1298, doi:10.1007/BF00987762
- ^ Monoclonal antibodies against tea polyphenols: A novel immunoassay to detect polyphenols in biological fluids. M. Gani, B. J. Mcguinness and A. P. Da Vies, Food and Agricultural Immunology, Volume 10, Issue 1 March 1998 , pages 13–22, doi:10.1080/09540109809354964
- ^ Walker, Richard B.; Everette, Jace D. (2009). "Comparative Reaction Rates of Various Antioxidants with ABTS Radical Cation". Journal of Agricultural and Food Chemistry 57 (4): 1156–61. doi:10.1021/jf8026765. PMID 19199590.
- ^ ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Roy Molay K, Koide, Motoki, Rao Theertham P, Okubo Tsutomu, Ogasawara Yutaka and Juneja Lekh R, International Journal of Food Sciences and Nutrition, Volume 61, Number 2, March 2010, pp. 109–124(16), PubMed, doi:10.3109/09637480903292601
- ^ Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay. Raquel Pulido, Laura Bravo and Fulgencio Saura-Calixto, J. Agric. Food Chem., 2000, 48 (8), pp 3396–3402, doi:10.1021/jf9913458
- ^ Inhibition of Human Low-Density Lipoprotein Oxidation in Relation to Composition of Phenolic Antioxidants in Grapes (Vitis vinifera). Anne S. Meyer, Ock-Sook Yi, Debra A. Pearson, Andrew L. Waterhouse and Edwin N. Frankel, J. Agric. Food Chem., 1997, 45 (5), pp 1638–1643, doi:10.1021/jf960721a
- ^ Mello, L; Sotomayor, Maria Del Pilar Taboada; Kubota, Lauro Tatsuo (2003). "HRP-based amperometric biosensor for the polyphenols determination in vegetables extract". Sensors and Actuators B: Chemical 96 (3): 636–45. doi:10.1016/j.snb.2003.07.008.
- ^ Regulation of polyphenol production in Vitis vinifera cell suspension cultures by sugars. F. Larronde, S. Krisa, A. Decendit, C. Chèze, G. Deffieux and J. M. Mérillon, Plant Cell Reports, Volume 17, Number 12, 946–950, doi:10.1007/s002990050515
Further reading
- Books
- Daayf, F. / Lattanzio, V. (eds.). Recent Advances in Polyphenol Research (Vol. 1). 2008. Wiley – Blackwell. ISBN 9781405158374
- Santos-Buelga, C. / Escribano-Bailon, M.T. / Lattanzio, V. (eds.). Recent Advances in Polyphenol Research (Vol. 2). 2010. Wiley – Blackwell. ISBN 9781405193993
- Review articles
- D’Archivo, M. et al. ”Polyphenols, dietary sources and bioavailability”, Annali dell'Istituto Superiore di Sanità (2007),43(4):348-361.
- Quideau, S. et al. ”Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis”, Angewandte Chemie International Edition (2011),50(3):586-621.
External links
- Other tools
- Phenol-Explorer, the first comprehensive and freely available electronic database on polyphenol content in foods.
- KNApSACK
- massbank.jp, a high resolution Mass Spectral Database
- PubChem, NCBI
- liberherbarum.com, the incomplete reference-guide to Herbal medicine, Copyright © Erik Gotfredsen.
- metabolomics.jp (English, Japanese)
- KEGG: Kyoto Encyclopedia of Genes and Genomes
- ChEBI
- Comparative Toxicogenomics Database for toxicity
Phytochemicals Misc: biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iGeneral Alkaloids Natural phenols PolyphenolsMonoterpenoids Diterpenoids Antibiotics ←Enzyme cofactors Classes Anthraquinones | chalconoids (C6-C3-C6) | Curcuminoids | Kavalactones | Naphthoquinones (C6-C4) | Phenylpropanoids (C6-C3) | Xanthonoids | IsocoumarinsSee also: PolyphenolsCategories:- Polyphenols
- Phytochemicals
- Dietary antioxidants
Wikimedia Foundation. 2010.





