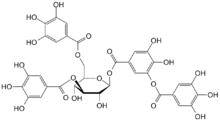
- Tannin
-
For other uses, see Tannin (demon).
A tannin (a.k.a. vegetable tannin, i.e. a type of biomolecule, as opposed to modern synthetic tannin) is an astringent, bitter plant polyphenolic compound that binds to and precipitates proteins and various other organic compounds including amino acids and alkaloids.
The term tannin (from tanna, an Old High German word for oak or fir tree, as in Tannenbaum) refers to the use of wood tannins from oak in tanning animal hides into leather; hence the words "tan" and "tanning" for the treatment of leather. However, the term "tannin" by extension is widely applied to any large polyphenolic compound containing sufficient hydroxyls and other suitable groups (such as carboxyls) to form strong complexes with proteins and other macromolecules.
The tannin compounds are widely distributed in many species of plants, where they play a role in protection from predation, and perhaps also as pesticides, and in plant growth growth regulation.[1] The astringency from the tannins is what causes the dry and puckery feeling in the mouth following the consumption of unripened fruit or red wine.[2] Likewise, the destruction or modification of tannins with time plays an important role in the ripening of fruit and the aging of wine.
Tannins have molecular weights ranging from 500 to over 3,000[3] (gallic acid esters) and up to 20,000 (proanthocyanidins). Tannins are incompatible with alkalis, gelatin, heavy metals, iron, lime water, metallic salts, strong oxidizing agents and zinc sulfate, since they form complexes and precipitate in aqueous solution.
Contents
Occurrence
Tannins are distributed in species throughout the plant kingdom. They are commonly found in both gymnosperms as well as angiosperms. Tannins are mainly physically located in the vacuoles or surface wax of plants. These storage sites keep tannins active against plant predators, but also keep some tannins from affecting plant metabolism while the plant tissue is alive; it is only after cell breakdown and death that the tannins are active in metabolic effects. Tannins are classified as ergastic substances, i.e., non-protoplasm materials found in cells.
Tannins are found in leaf, bud, seed, root, and stem tissues. An example of the location of the tannins in stem tissue is that they are often found in the growth areas of trees, such as the secondary phloem and xylem and the layer between the cortex and epidermis. Tannins may help regulate the growth of these tissues.
There may be a loss in the bio-availability of still other tannins in plants due to birds, pests, and other pathogens.[4]
The most abundant polyphenols are the condensed tannins, found in virtually all families of plants, and comprising up to 50% of the dry weight of leaves.
Presence in soils
The convergent evolution of tannin-rich plant communities has occurred on nutrient-poor acidic soils throughout the world. Tannins were once believed to function as anti-herbivore defenses, but more and more ecologists now recognize them as important controllers of decomposition and nitrogen cycling processes. As concern grows about global warming, there is great interest to better understand the role of polyphenols as regulators of carbon cycling, in particular in northern boreal forests.
Presence in water and wood
The leaching of highly water soluble[5] tannins from decaying vegetation and leaves along a stream may produce what is known as a blackwater river. Water flowing out of bogs has a characteristic brown color from dissolved peat tannins. The presence of tannins (and/or humic acid) in well water can make it smell bad or taste bitter, but not be unsafe to drink.[6]
Tannins leaching from an unprepared driftwood decoration in an aquarium can cause pH lowering and coloring of the water to a tea-like tinge. A remedy is to boil the wood in water several times (which will also darken and waterlog the wood, i.e., make it sink), discarding the water each time. Using peat as an aquarium substrate can lead to the same problem.
Many hours of boiling the driftwood may need to be followed by many weeks or months of constant soaking and many water changes before the water will stay clear. Adding baking soda to the water to raise its pH level will accelerate the process of leaching, as the more alkaline solution can draw out tannic acid from the wood faster than the pH-neutral water.[7]
Softwoods, while in general much lower in tannins than hardwoods,[8] are usually not recommended for use in an aquarium[9] so using a hardwood with a very light color, indicating a low tannin content, can be an easy way to avoid tannins. Tannic acid is brown in color, so in general white woods have a low tannin content. Woods with a lot of yellow, red, or brown coloration to them (like southern yellow pine, cedar, redwood, red oak, etc.) tend to contain a lot of tannin.[10] Finnish hardwoods, like birch and aspen, do not contain tannins.[11]
Classes of tannins
There are three major classes of tannins:[12]
Base Unit: 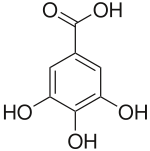
Gallic acid 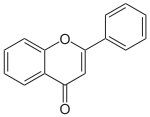
Flavone 
Phloroglucinol Class/Polymer: Hydrolyzable tannins Non-Hydrolyzable
or condensed tanninsPhlorotannins Sources Plants Plants Brown algae Pseudo tannins
Pseudo tannins are low molecular weight compounds associated with other compounds. They do not answer gold beater skin test unlike hydrolysable and condensed tannins.[12] (When gold beater skin or ox skin is dipped in HCl & treated with 1% FeSO4 solution, after washing with water it gives a blue / black colour). They are found in tea or coffee[13] or in:[14]
- Gallic acid: rhubarb
- flavan-3-ols (Catechins): Acacia, catechu, cocoa, guarana
- Chlorogenic acid: Nux-vomica, coffee, mate
- Ipecacuanhic acid: Carapichea ipecacuanha
Nutrition
Tannins have traditionally been considered antinutritional but it is now known that their beneficial or antinutritional properties depend upon their chemical structure and dosage. The new technologies used to analyze molecular and chemical structures have shown that a division into condensed and hydrolyzable tannins is far too simplistic.[15] Recent studies have demonstrated that products containing chestnut tannins included at low dosages (0.15–0.2 %) in the diet can be beneficial.[16] Some studies suggest that chestnut tannins have been shown to have positive effects on silage quality in the round bale silages, in particular reducing NPNs (non protein nitrogen) in the lowest wilting level.[17] Improved fermentability of soya meal nitrogen in the rumen has also been reported by F. Mathieu and J.P. Jouany (1993).[18] Studies by S. Gonzalez et al. (2002)[19] on in vitro ammonia release and dry matter degradation of soybean meal comparing three different types of tannins (quebracho, acacia and chestnut) demonstrated that chestnut tannins are more efficient in protecting soybean meal from in vitro degradation by rumen bacteria.
Condensed tannins inhibit herbivore digestion by binding to consumed plant proteins and making them more difficult for animals to digest, and by interfering with protein absorption and digestive enzymes (for more on that topic, see plant defense against herbivory).
Many tannin-consuming animals secrete a tannin-binding protein (mucin) in their saliva. Tannin-binding capacity of salivary mucin is directly related to its proline content. Advantages in using salivary proline-rich proteins (PRPs) to inactivate tannins are:- PRPs inactivate tannins to a greater extent than do dietary proteins; this results in reduced fecal nitrogen losses
- PRPs contain non specific nitrogen and nonessential amino acids; this makes them more convenient for an animal to exploit rather than using up valuable dietary protein
Drinks with tannins
Main articles: Tannins in tea and Tannins in wineBeer
In addition to the alpha acids extracted from hops to provide bitterness in beer, condensed tannins are also present. These originate both from the malt and hops. Especially in Germany, trained brewmasters consider the presence of tannins as a flaw. However, in some styles, the presence of this astringency is acceptable or even desired, as, for example, in a Flanders red ale.
In lager type beers, the tannins can form a precipitate with specific haze forming proteins in the beer resulting in turbidity at low temperature. This chill haze can be prevented by removing part of the tannins or part of the haze forming proteins. Tannins are removed using PVPP, haze forming proteins by using silica or tannic acid.[20]
Fruit juices
Although citrus fruits do not themselves contain tannins, orange-colored juices often contain food dyes with tannins. Apple juice, grape juices and berry juices are all high in tannins. Sometimes tannins are even added to juices and ciders to create a more astringent feel to the taste.
Drinks without tannins
Coffee
Tannins were not found in any bean sample, and, in contrast to previous reports, hydrolysable tannins sensu stricto were not detected in pulp. The presence of soluble condensed tannins in Coffea arabica pulp was confirmed at approximately 1%.[21]
Food items with tannins
Fruits
Pomegranates
Pomegranates contain a diverse array of tannins, in particular hydrolysable tannins. The most abundant of pomegranate tannins are called punicalagins. Punicalagins have a molecular weight of 1038 and are the largest molecule found intact in rat plasma after oral ingestion[22] and were found to show no toxic effects in rats who were given a 6% diet of punicalagins for 37 days.[23] Punicalagins are also found to be the major component responsible for pomegranate juice's antioxidant and health benefits.[24]
Several dietary supplements and nutritional ingredients are available that contain extracts of whole pomegranate and/or are standardized to punicalagins, the marker compound of pomegranate. Extracts of pomegranate are also Generally Recognized as Safe (GRAS) by the United States Food and Drug Administration. It has been recommended to look for pomegranate ingredients that mimic the polyphenol ratio of the fruit, as potent synergistic effects have been observed in 'natural spectrum' extracts, especially pomegranate concentrate normalized to punicalagins.[25]
Persimmons
Some persimmons are highly astringent and therefore inedible when they are not extremely ripe (to be specific, the Korean, American, and Hachiya or Japanese). This is due to the high level of tannins, and if eaten by humans (and many other animals), the mouth will become completely dry, yet the saliva glands will continue to secrete saliva, which cannot affect the tannin-laced food.[clarification needed]
Berries
Most berries, such as cranberries,[26] strawberries and blueberries,[27] contain both hydrolyzable and condensed tannins.
Nuts
Nuts that can be consumed raw such as hazelnuts, walnuts, and pecans contain high amounts of tannins. Almonds feature a lower content. Tannin concentration in the crude extract of these nuts did not directly translate to the same relationships for the condensed fraction.[28] Acorns contain such high concentrations of tannins that they need to be processed before they can be consumed safely.
The areca nut also contains tannin, which contributes to its antibacterial properties.
Smoked foods
Tannins from the wood of mesquite, cherry, oak, and other woods used in smoking are present on the surface of smoked fish and meat.
Herbs and spices
Cloves, tarragon, cumin, thyme, vanilla, and cinnamon all contain tannins.[29]
Legumes
Most legumes contain tannins. Red-colored beans contain the most tannins, and white-colored beans have the least. Peanuts without shells have a very low tannin content. Chickpeas (garbanzo beans) have a smaller amount of tannins.[30]
Chocolate
Chocolate liquor contains about 6% tannins.[31]
Toxicity
Tannins have been shown to precipitate proteins,[3] which in some ruminant animals inhibits the absorption of nutrients from high-tannin grains such as sorghum.
In sensitive individuals, a large intake of tannins may cause bowel irritation, kidney irritation, liver damage, irritation of the stomach, and gastrointestinal pain. With the exception of tea, long-term and/or excessive use of herbs containing high concentrations of tannins is not recommended. A correlation has been made between esophageal or nasal cancer in humans and regular consumption of certain herbs with high tannin concentrations.[32]
Many plants employ tannins to deter animals. It has not been determined whether tannin was produced for another purpose, e.g. as pesticide, or whether it evolved specifically for the purpose of inhibiting predation.[33] Animals that consume excessive amounts of these plants fall ill or die. Acorns are a well-known problem in cattle breeding. The lethal dose is said to be around 6% of the animal's body weight. This is only an approximate figure, since acorns from red oak were shown to contain on average two to four times the tannins of those from white oak.[34] Some deer and moose were found to have perished due to ingesting acorns. Symptoms include ataxia and shortness of breath. Some animals, like squirrels and mule deer, possess the ability to consume high concentrations of tannins without ill effects. Humans would usually find the bitter taste of foods containing high amounts of tannins unpalatable. (Some humans were found to be unable to taste bitter foods.[citation needed]) Tannins are leached from acorns before they are used for human consumption.
Metal chelation
If ingested in excessive quantities, tannins inhibit the absorption of minerals such as iron, which may, if prolonged, lead to anemia.[35] This is because tannins are metal ion chelators[36] and tannin-chelated metal ions are not bioavailable. Tannins only reduce the bioavailability of plant sources of iron, also known as non-heme. Animal sources, or heme iron absorption will not be affected by tannins. Tannic acid does not affect absorption of other trace minerals such as zinc, copper, and manganese in rats.[37]
Tannins interfere with iron absorption through a complex formation with iron when it is in the gastrointestinal lumen, which decreases the bioavailability of iron. There is an important difference in the way in which the phenolic compounds interact with different hydroxylation patterns (gallic acid, catechin, chlorogenic acid) and the effect on iron absorption. The content of the iron-binding galloyl groups may be the major determinant of the inhibitory effect of phenolic compounds. However, condensed tannins do not interfere with iron absorption.[35]
In order to prevent these problems, it is advised to drink tea and coffee between meals, not during. Foods rich in vitamin C help neutralize tannin's effects on iron absorption. Adding lemon juice to tea will reduce the negative effect of tannins in iron absorption as well. Adding milk to coffee and tea has very little to no influence on the inhibitory effect of tannins.[38]
Tannin market
Tannin production began at the beginning of the 19th century with the industrial revolution, to produce tanning material for the need for more leather. Before that time, processes used plant material and were long (up to six months).
There has been a collapse in the vegetable tannin market in the 1950s-1960s, due to the appearance of synthetic tannins, due the scarity of vegetable tannins during World War II. At that time, many small tannin industry sites closed.[39] Vegetable tannins are estimated to be used for the production of 10-20% of the global leather production.
The cost of the final product depends on the method used to extract the tannins, in particular the use of solvents, alkali and other chemicals used (for instance glycerin). For large quantities, the most cost-effective method is hot water extraction.
Tannic acid is used worldwide as clarifying agent in alcoholic drinks and as aroma ingredient in both alcoholic and soft drinks or juices. Tannins from different botanical origins also find extensive uses in the wine industry.
Uses
Tannins are an important ingredient in the process of tanning leather. Oak bark, mimosa, chestnut and quebracho tree have traditionally been the primary source of tannery tannin, though inorganic tanning agents are also in use today and account for 90% of the world's leather production.[40]
Tannins produce different colors with ferric chloride (either blue, blue black, or green to greenish-black) according to the type of tannin. Iron gall ink is produced by treating a solution of tannins with iron(II) sulfate.[citation needed]
Tannin is a component in a type of industrial particleboard adhesive developed jointly by the Tanzania Industrial Research and Development Organization and Forintek Labs Canada.[41] Pinus radiata tannins has been investigated for the production of wood adhesives.[42]
Condensed tannins, i.e. quebracho tannin, and Hydrolyzable tannins, i.e., chestnut tannin, appear to be able to substitute a high proportion of synthetic phenol in phenol-formaldehyde resins for wood particleboard.
Tannins can be used for production of anti-corrosive primer, sold under brand name-Nox Primer for treatment of rusted steel surfaces prior to painting, rust converter to transform oxidized steel into a smooth sealed surface and rust inhibitor.
The use of resins made of tannins has been investigated to remove mercury and methylmercury from solution.[43] Immobilized tannins have been tested to recover uranium from seawater.[44]
Medical uses and potential
See also: Wine and healthTannins can also be effective in protecting the kidneys. When incubated with red grape juice and red wines with a high content of condensed tannins, the poliovirus, herpes simplex virus, and various enteric viruses are inactivated.[45]
Tannins have shown potential antiviral,[46] antibacterial[47] and antiparasitic effects.[48]
It is believed that tannins isolated from the stem bark of Myracrodruon urundeuva may have neuroprotective functions capable of reversing 6-hydroxydopamine-induced toxicity. The plant has shown promise as a potential therapeutic agent, which may be beneficial in patients with neurological disease.[49] Souza et al. discovered that the tannins isolated from the stem bark also have anti-inflammatory and antiulcer activity in rodents, showing a strong antioxidant property with possible therapeutic applications.[50]
Foods rich in tannins can be used in the treatment of HFE hereditary hemochromatosis, a hereditary disease characterized by excessive absorption of dietary iron, resulting in a pathological increase in total body iron stores.
Producers
There are three main producers of tannins in the world:
- Ajinomoto OmniChem NaturalSpecialities, a subsidiary of the Ajinomoto group, is specializing in the tannin production[51]
- Silva Team produces tannins for leather tanning, oenology or other uses[52]
- Unitán, an Argentine company, produces quebracho tannins.
Following companies are not producers but blenders of tannins for specific applications:
- AEB group, an Italian company based in Brescia, blends & markets tannins for oenology applications[53]
- Enartis, an Italian company based in San Martino, develops and sells oenological tannins[54]
See also
References
Notes
- ^ Katie E. Ferrell; Thorington, Richard W. (2006). Squirrels: the animal answer guide. Baltimore: Johns Hopkins University Press. p. 91. ISBN 0-8018-8402-0.
- ^ McGee, Harold (2004). On food and cooking: the science and lore of the kitchen. New York: Scribner. p. 714. ISBN 0-684-80001-2.
- ^ a b Bate-Smith and Swain (1962). "Flavonoid compounds". In Florkin M., Mason H.S.. Comparative biochemistry. III. New York: Academic Press. pp. 75–809.
- ^ Kadam, S. S.; Salunkhe, D. K.; Chavan, J. K. (1990). Dietary tannins: consequences and remedies. Boca Raton: CRC Press. p. 177. ISBN 0-8493-6811-1.
- ^ Tannic acid (Wikipedia)
- ^ Tannins, lignins and humic acids in well water on www.gov.ns.ca
- ^ Preparing Driftwood for Your Freshwater Aquarium
- ^ Polyflavonoid tannins — a main cause of soft-rot failure in CCA-treated timber
- ^ Driftwood Do's & Don'ts
- ^ Tannin and hardwood flooring
- ^ Characterisation of thermally modified hard- and softwoods by 13C CPMAS NMR
- ^ a b Notes on Tannins from PharmaXChange.info
- ^ Tannins, Unknown author
- ^ Ashutosh Kar (2003). Pharmacognosy And Pharmacobiotechnology. New Age International. pp. 44–. ISBN 9788122415018. http://books.google.com/books?id=PlMi4XvHCYoC&pg=PA44. Retrieved 31 January 2011.
- ^ Muller-Harvey I, McAllan AB (1992). "Tannins: Their biochemistry and nutritional properties". Adv. Plant Cell Biochem. And Biotechnol. 1: 151–217.
- ^ Schiavone A, Guo K, Tassone S, et al. (March 2008). "Effects of a natural extract of chestnut wood on digestibility, performance traits, and nitrogen balance of broiler chicks". Poult. Sci. 87 (3): 521–7. doi:10.3382/ps.2007-00113. PMID 18281579. http://ps.fass.org/cgi/pmidlookup?view=long&pmid=18281579.
- ^ Tabacco E, Borreani G, Crovetto GM, Galassi G, Colombo D, Cavallarin L (1 December 2006). "Effect of chestnut tannin on fermentation quality, proteolysis, and protein rumen degradability of alfalfa silage". J. Dairy Sci. 89 (12): 4736–46. doi:10.3168/jds.S0022-0302(06)72523-1. PMID 17106105. http://jds.fass.org/cgi/pmidlookup?view=long&pmid=17106105.
- ^ Mathieu F, Jouany JP (1993). "Effect of chestnut tannin on the fermentability of soyabean meal nitrogen in the rumen". Ann Zootech 42: 127. doi:10.1051/animres:19930210.
- ^ González S, Pabón ML, Carulla J (2002). "Effects of tannins on in vitro ammonia release and dry matter degradation of soybean meal". Arch. Latinoam. Prod. Anim. 10 (2): 97–101.
- ^ http://www.natural-specialities.com/natural-specialities/PDF/Applications/BR02%20overview%20fact%20sheet%20version%202.1.pdf
- ^ Clifford M.N., Ramirez-Martinez J.R. (1991). "Tannins in wet-processed coffee beans and coffee pulp". Food Chemistry 40 (2): 191–200. doi:10.1016/0308-8146(91)90102-T. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T6R-49NR0X7-1XV&_user=10&_coverDate=12%2F31%2F1991&_rdoc=1&_fmt=high&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=b61362d5024028d01bdcef6377c13be8.
- ^ Scalbert A, Morand C, Manach C, Rémésy C (August 2002). "Absorption and metabolism of polyphenols in the gut and impact on health". Biomed. Pharmacother. 56 (6): 276–82. doi:10.1016/S0753-3322(02)00205-6. PMID 12224598.
- ^ Cerdá B, Cerón JJ, Tomás-Barberán FA, Espín JC (May 2003). "Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic". J. Agric. Food Chem. 51 (11): 3493–501. doi:10.1021/jf020842c. PMID 12744688.
- ^ Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA (October 2000). "Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing". J. Agric. Food Chem. 48 (10): 4581–9. doi:10.1021/jf000404a. PMID 11052704.
- ^ Seeram NP, Adams LS, Henning SM, et al. (June 2005). "In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice". J. Nutr. Biochem. 16 (6): 360–7. doi:10.1016/j.jnutbio.2005.01.006. PMID 15936648. http://linkinghub.elsevier.com/retrieve/pii/S0955-2863(05)00019-7.
- ^ Vattem DA, Ghaedian R, Shetty K (2005). "Enhancing health benefits of berries through phenolic antioxidant enrichment: focus on cranberry". Asia Pac J Clin Nutr 14 (2): 120–30. PMID 15927928. http://www.nupro.net/science/enhnce%20berry%20phenoli.pdf.
- ^ Puupponen-Pimiä R, Nohynek L, Meier C, et al. (April 2001). "Antimicrobial properties of phenolic compounds from berries". J. Appl. Microbiol. 90 (4): 494–507. doi:10.1046/j.1365-2672.2001.01271.x. PMID 11309059. http://www.blackwell-synergy.com/links/doi/10.1046/j.1365-2672.2001.01271.x/abs/.
- ^ http://google.com/search?q=cache:K-qF4vdf8a8J:www.icomst.helsinki.fi/icomst2008/Paper%2520CD/General%2520speakers%2Bposters-3p%2520papers/Session2/2B/2B.3.Amarowicz.pdf+tannin+%22nuts%22&hl=en&ct=clnk&cd=10&gl=us[dead link]
- ^ Navia, Jeanette. “Could Tannins Explain Classic Migraine Triggers?” 1988
- ^ Reed JD (1 May 1995). "Nutritional toxicology of tannins and related polyphenols in forage legumes". J. Anim. Sci. 73 (5): 1516–28. PMID 7665384. http://jas.fass.org/cgi/pmidlookup?view=long&pmid=7665384.
- ^ Robert L. Wolke; Marlene Parrish (29 March 2005). What Einstein told his cook 2: the sequel : further adventures in kitchen science. W. W. Norton & Company. p. 433. ISBN 9780393058697. http://books.google.com/books?id=jGYMiTMhp9UC&pg=PA433.
- ^ Elvin-Lewis, Memory P. F.; Lewis, Walter Hepworth (1977). Medical botany: plants affecting man's health. New York: Wiley. ISBN 0-471-53320-3.
- ^ Katie E. Ferrell; Thorington, Richard W. (2006). Squirrels: the animal answer guide. Baltimore: Johns Hopkins University Press. p. 91. ISBN 0-8018-8402-0.
- ^ Wood MD (2005). "Tannin and Lipid Content of Acorns in Scatterhoards and Larderhoards". Northeastern Naturalist 12 (4): 463–72. doi:10.1656/1092-6194(2005)012[0463:TALCOA]2.0.CO;2. "Figure 2: Mean tannin levels of red oak and white oak acorns retrieved after a period of storage."
- ^ a b Brune M, Rossander L, Hallberg L (August 1989). "Iron absorption and phenolic compounds: importance of different phenolic structures". Eur J Clin Nutr 43 (8): 547–57. PMID 2598894.
- ^ Karamać M (December 2009). "Chelation of Cu(II), Zn(II), and Fe(II) by tannin constituents of selected edible nuts". Int J Mol Sci 10 (12): 5485–97. doi:10.3390/ijms10125485. PMC 2802006. PMID 20054482. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2802006.
- ^ Afsana K, Shiga K, Ishizuka S, Hara H (1 November 2003). "Ingestion of an indigestible saccharide, difructose anhydride III, partially prevents the tannic acid-induced suppression of iron absorption in rats". J. Nutr. 133 (11): 3553–60. PMID 14608073. http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=14608073.
- ^ Hurrell RF, Reddy M, Cook JD (April 1999). "Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages". Br. J. Nutr. 81 (4): 289–95. PMID 10999016. http://journals.cambridge.org/abstract_S0007114599000537.
- ^ The Status of Mangrove Ecosystems: Trends in the Utilisation and Management of Mangrove Resources. D. Macintosh and S. Zisman
- ^ Marion Kite; Roy Thomson (2006). Conservation of leather and related materials. Butterworth-Heinemann. p. 23. ISBN 9780750648813. http://books.google.com/books?id=62zZy2B4ehQC.
- ^ Bisanda E.T.N., Ogola W.O., Tesha J.V. (August 2003). "Characterisation of tannin resin blends for particle board applications". Cement and Concrete Composites 25 (6): 593–8. doi:10.1016/S0958-9465(02)00072-0. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TWF-47283Y6-1&_user=4296857&_rdoc=1&_fmt=&_orig=search&_sort=d&_docanchor=&view=c&_rerunOrigin=google&_acct=C000012518&_version=1&_urlVersion=0&_userid=4296857&md5=66af73623c18c75f87a46769f57a2496.
- ^ Li, Jingge; Maplesden, Frances (1998). "Commercial production of tannins from radiata pine bark for wood adhesives" (PDF). IPENZ Transactions 25 (1/EMCh). http://www.ipenz.org.nz/ipenz/publications/transactions/Transactions98/emch/7li.PDF.
- ^ Torres J., Olivares S., De La Rosa D., Lima L., Martínez F., Munita C.S., Favaro D.I.T. (1999). "Removal of mercury(II) and methylmercury from solution by tannin adsorbents". Journal of Radioanalytical and Nuclear Chemistry 240 (1): 361–5. doi:10.1007/BF02349180. http://www.springerlink.com/content/nu6j27t61654t944/.
- ^ Takashi Sakaguchia, Akira Nakajimaa (June 1987). "Recovery of Uranium from Seawater by Immobilized Tannin". Separation Science and Technology 22 (6): 1609–23. doi:10.1080/01496398708058421. http://www.informaworld.com/smpp/content~db=all~content=a762595700.
- ^ Bajaj, Y. P. S. (1988). Medicinal and aromatic plants. Biotechnology in agriculture and forestry. 24. Berlin: Springer-Verlag. ISBN 0-387-56008-4.
- ^ Lü L, Liu SW, Jiang SB, Wu SG (February 2004). "Tannin inhibits HIV-1 entry by targeting gp41". Acta Pharmacol. Sin. 25 (2): 213–8. PMID 14769212.
- ^ Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K (October 2001). "Antibacterial action of several tannins against Staphylococcus aureus". J. Antimicrob. Chemother. 48 (4): 487–91. doi:10.1093/jac/48.4.487. PMID 11581226. http://jac.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=11581226.
- ^ Kolodziej H, Kiderlen AF (September 2005). "Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitised RAW 264.7 cells". Phytochemistry 66 (17): 2056–71. doi:10.1016/j.phytochem.2005.01.011. PMID 16153409. http://linkinghub.elsevier.com/retrieve/pii/S0031-9422(05)00012-9.
- ^ Nobre-Junior, Helio V. et al. (2007). "Neuroprotective Actions of Tannins from Myracrodruon urundeuva on 6-Hydroxydopamine-Induced Neuronal Cell Death". Journal of Herbs, Spices & Medicinal Plants (Haworth Press) 13 (2). http://www.haworthpress.com/store/Toc_views.asp?sid=TEJBLR1GTE9P9L4KKH98M4BFKMUS112B&TOCName=J044v13n02%5FTOC&desc=Volume%3A%2013%20Issue%3A%202. Retrieved 8 November 2007.
- ^ Souza, S. M. C. et al.; Aquino, LC; Milach Jr, AC; Bandeira, MA; Nobre, ME; Viana, GS (2006). "Antiinflammatory and antiulcer properties of tannins from Myracrodruon urundeuva Allemão (Anacardiaceae) in Rodents". Phytotherapy Research (John Wiley & Sons) 21 (3): 220–225. doi:10.1002/ptr.2011. PMID 17154231.
- ^ www.natural-specialities.com
- ^ Products on www.silvateam.com
- ^ Oenological tannins on www.aeb-group.com
- ^ Tannins on www.enartis.com
- Calvi L, Mwalongo GCJ, Mwingira BA, Riedl B, Shields JA (1995). "Characterization of Wattle-Tannin-Based Adhesives for Tanzania". Holzforchung 49 (2).
External links
- Tannins: fascinating but sometimes dangerous molecules
- Tannin ChemistryPDF (1.41 MB)
- Haslam, Edwin (1989). Plant polyphenols: vegetable tannins revisited. CUP Archive. ISBN 9780521321891. http://books.google.com/books?id=Zyc9AAAAIAAJ.
Types of tannins Types Hydrolysable | Condensed | Phlorotannins | Flavono-ellagitannins (complex tannins)Tannin uses Other Tannin sourcesMiscellaneous Pseudo tannins | Synthetic tannins
Wikimedia Foundation. 2010.