- Resorcinol
-
Resorcinol 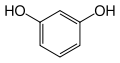
 Benzene-1,3-diolOther namesResorcin
Benzene-1,3-diolOther namesResorcin
m-dihydroxybenzene; 1,3-benzenediol; 1,3-Dihydroxybenzene; 3-Hydroxyphenol; m-hydroquinone; m-benzenediol; 3-hydroxycyclohexadien-1-oneIdentifiers CAS number 108-46-3 
PubChem 5054 ChemSpider 4878 
UNII YUL4LO94HK 
UN number 2876 KEGG D00133 
ChEBI CHEBI:27810 
ChEMBL CHEMBL24147 
Jmol-3D images Image 1 - c1cc(cc(c1)O)O
Properties Molecular formula C6H6O2 Molar mass 110.1 g/mol Appearance White solid Density 1.28 g/cm3, solid Melting point 110 °C, 383 K, 230 °F
Boiling point 277 °C, 550 K, 531 °F
Solubility in water 110 g/100 mL at 20 °C Acidity (pKa) 9.15[1] Hazards EU classification Harmful (Xn)
Dangerous for
the environment (N) (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Resorcinol (or resorcin) is a dihydroxy benzene. It is the 1,3-isomer of benzenediol with the formula C6H4(OH)2.
Contents
Nomenclature
Benzene-1,3-diol is the name recommended by the International Union of Pure and Applied Chemistry (IUPAC) in its 1993 Recommendations for the Nomenclature of Organic Chemistry.[2]
Production
It is produced when any of a large number of resins (e.g., galbanum, asafoetida, etc.) are melted with potassium hydroxide, or by the distillation of Brazilwood extract. It may be prepared synthetically by melting 3-iodophenol, phenol-3-sulfonic acid, or benzene-1,3-disulfonic acid with potassium carbonate; by the action of nitrous acid on 3-aminophenol; or by the action of 10% hydrochloric acid on 1,3-diaminobenzene.[3] Many ortho- and para-compounds of the aromatic series (for example, the bromophenols, benzene-para-disulfonic acid) also yield resorcinol on fusion with potassium hydroxide.
Properties
Resorcinol crystallizes from benzene as colorless needles that are readily soluble in water, alcohol, and ether, but insoluble in chloroform and carbon disulfide. It reduces Fehling's solution and ammoniacal silver solutions. It does not form a precipitate with lead acetate[disambiguation needed
 ] solution, as the isomeric pyrocatechol does. Iron(III) chloride colors its aqueous solution a dark-violet, and bromine water precipitates tribromoresorcin. These properties are what give it its use as a colouring agent for certain chromatography experiments. Sodium amalgam reduces it to dihydroresorcin, which when heated to 150 to 160 °C with concentrated barium hydroxide solution gives γ-acetylbutyric acid (D. Vorlgnder); when fused with potassium hydroxide, resorcinol yields phloroglucin, pyrocatechol, and diresorcin. It condenses with acids or acid chlorides, in the presence of dehydrating agents, to oxyketones, e.g. with zinc chloride and glacial acetic acid at 145 °C it yields resacetophenone (HO)2C6H3~CO.CH3.[4] With the anhydrides of dibasic acids, it yields fluoresceins. When heated with calcium chloride—ammonia to 200 °C it yields meta-dioxydiphenylamine.[5] With sodium nitrite it forms a water-soluble blue dye, which is turned red by acids, and is used as an indicator, under the name of lacmoid.[6] It condenses readily with aldehydes, yielding with formaldehyde, on the addition of catalytic hydrochloric acid, methylene diresorcin [(HO)C6H3(O)]2•CH2. Reaction with chloral hydrate in the presence of potassium bisulfate yields the lactone of tetra-oxydiphenyl methane carboxylic acid.[7] In alcoholic solution it condenses with sodium acetoacetate to form 4-methylumbelliferone.[8]
] solution, as the isomeric pyrocatechol does. Iron(III) chloride colors its aqueous solution a dark-violet, and bromine water precipitates tribromoresorcin. These properties are what give it its use as a colouring agent for certain chromatography experiments. Sodium amalgam reduces it to dihydroresorcin, which when heated to 150 to 160 °C with concentrated barium hydroxide solution gives γ-acetylbutyric acid (D. Vorlgnder); when fused with potassium hydroxide, resorcinol yields phloroglucin, pyrocatechol, and diresorcin. It condenses with acids or acid chlorides, in the presence of dehydrating agents, to oxyketones, e.g. with zinc chloride and glacial acetic acid at 145 °C it yields resacetophenone (HO)2C6H3~CO.CH3.[4] With the anhydrides of dibasic acids, it yields fluoresceins. When heated with calcium chloride—ammonia to 200 °C it yields meta-dioxydiphenylamine.[5] With sodium nitrite it forms a water-soluble blue dye, which is turned red by acids, and is used as an indicator, under the name of lacmoid.[6] It condenses readily with aldehydes, yielding with formaldehyde, on the addition of catalytic hydrochloric acid, methylene diresorcin [(HO)C6H3(O)]2•CH2. Reaction with chloral hydrate in the presence of potassium bisulfate yields the lactone of tetra-oxydiphenyl methane carboxylic acid.[7] In alcoholic solution it condenses with sodium acetoacetate to form 4-methylumbelliferone.[8]In addition to electrophilic aromatic addition, resorcinol (and other poly-ols)undergo nucleophilic substitution via the enone form. With concentrated nitric acid, in the presence of cold concentrated sulfuric acid, it yields trinitro-resorcin (styphnic acid), which forms yellow crystals, exploding violently on rapid heating.
Occurrence
Parts of a molecule of catechin, another natural compound that is present in tea, has the resorcinol skeleton structure in it.
Applications
Medical
Used externally, it is an antiseptic and disinfectant, and is used 5 to 10% in ointments in the treatment of chronic skin diseases such as psoriasis, hidradenitis suppurativa, and eczema of a sub-acute character.[citation needed] It is present in over-the-counter topical acne treatments at 2% or less concentration, and in prescription treatments at higher concentrations.[citation needed] Weak, watery solutions of resorcinol (25 to 35 g/kg) are useful in allaying the itching in erythematous eczema.[citation needed] A 2% solution used as a spray has been used with marked effect in hay fever and in whooping cough.[citation needed] In the latter disease 0.6 mL of the 2% solution has been given internally. It can be included as an anti-dandruff agent in shampoo or in sunscreen cosmetics.[citation needed] It has also been employed in the treatment of gastric ulcers in doses of 125 to 250 mg in pills, and is said to be analgesic and haemostatic in its action.[citation needed] In large doses, it is a poison, causing giddiness, deafness, salivation, sweating, and convulsions. It is also worked up in certain medicated soaps.[citation needed] Monoacetylresorcinol, C6H4(OH)(O-COCH3), is used under the name of euresol.[citation needed] Resorcinol is one of the main active ingredients in products like Resinol and Vagisil. Resorcinol is also an active ingredient in certain Clearasil products.
Chemical
Resorcinol is also used as a chemical intermediate for the synthesis of pharmaceuticals and other organic compounds. It is used in the production of diazo dyes and plasticizers and as a UV absorber in resins.
An emerging use of resorcinol is as a template molecule in supramolecular chemistry. The -OH groups on resorcinol form hydrogen bonds to target molecules, holding them in the proper orientation for a reaction. Many such reactions are able to be carried out in the solid state, thereby reducing or eliminating the use of solvents that may be harmful to the environment. (see Green chemistry)
Resorcinol is an analytical reagent for the qualitative determinaion of ketoses (Seliwanoff's test).
Resorcinol is the starting material for resorcinarene molecules and the initiating explosive lead styphnate (Reference: Army TM 9-1300-214, p. 7–12).
Resorcinol reacts with formaldehyde to form a thermoset resin, which can form the basis of an aerogel.
Related compounds
Resazurin, C12H7NO4, obtained by the action of nitrous acid on resorcinol,[9] forms small dark red crystals possessing a greenish metallic glance. When dissolved in concentrated sulfuric acid and warmed to 210 °C, the solution on pouring into water yields a precipitate of resorufin, C12H7NO3, an oxyphenoxazone, which is insoluble in water, but is readily soluble in hot concentrated hydrochloric acid, and in solutions of caustic alkalis. The alkaline solutions are of a rose-red color and show a cinnabar-red fluorescence. A tetrabromresorufin is used as a dye-stuff under the name of Fluorescent Resorcin Blue.
Thioresorcinol is obtained by the action of zinc and hydrochloric acid on the chloride of benzene meta-disulfonic acid. It melts at 27 °C and boils at 243 °C. Resorcinol disulfonic acid (HO)2C6H2(HSO3)2, is a deliquescent mass obtained by the action of sulfuric acid on resorcin.[10] It is easily soluble in water and decomposes when heated to 100 °C.
Alkylresorcinols are a class of phenolic lipids fund in wheat or rye brans.
See also
- Resorcinol glue
References
- ^ Gawron, O., Duggan, M., Grelechi, C., J. Anal. Chem., 1952, 24, 969.
- ^ Panico, R.; & Powell, W. H. (Eds.) (1994). A Guide to IUPAC Nomenclature of Organic Compounds 1993. Oxford: Blackwell Science. ISBN 0-632-03488-2.
- ^ Meyer, J (1897). Ber 30: 2569.
- ^ M. Nencki and N. Sieber, Jour. prak. Chem., 1881, 23, p. 147
- ^ A. Seyewitz, Bull. Soc. Chins., 1890, 3, p. 811
- ^ M. C. Traub and C. Hock, Ber., 1884, 17, p. 2615
- ^ J. T. Hewitt and F. G. Pope, Jour. C/tern. Soc., 1897, 75, p. 1084
- ^ A. Michael, Jour. prak. Chem., 1888, 37, 470
- ^ Weselsky, P; Benedikt, R (1880). Monats. f: 889.
- ^ Fischer, H (1881). Monats. 2: 321.
External links
- International Chemical Safety Card 1033
- NIOSH Pocket Guide to Chemical Hazards
- IARC Monograph: "Resorcinol"
- IUPAC Nomenclature of Organic Chemistry (online version of the "Blue Book")
 This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press.Categories:
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press.Categories:- Natural resorcinols
- IARC Group 3 carcinogens
- Endocrine disruptors
Wikimedia Foundation. 2010.
