- Fractionation
-
See also: Fractionated spacecraft
Fractionation is a separation process in which a certain quantity of a mixture (solid, liquid, solute, suspension or isotope) is divided up in a number of smaller quantities (fractions) in which the composition changes according to a gradient. Fractions are collected based on differences in a specific property of the individual components. A common trait in fractionations is the need to find an optimum between the amount of fractions collected and the desired purity in each fraction. Fractionation makes it possible to isolate more than two components in a mixture in a single run. This property sets it apart from other separation techniques.
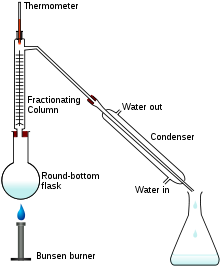 Fractional distillation apparatus using a Liebig condenser. A conical flask is used as a receiving flask. Here the distillation head and fractionating column are combined in one piece.
Fractional distillation apparatus using a Liebig condenser. A conical flask is used as a receiving flask. Here the distillation head and fractionating column are combined in one piece.
Fractionation is widely employed in many branches of science and technology. Mixtures of liquids and gases are separated by fractional distillation by difference in boiling point. Fractionation of components also takes place in column chromatography by a difference in affinity between stationary phase and the mobile phase. In fractional crystallization and fractional freezing, chemical substances are fractionated based on difference in solubility at a given temperature. In cell fractionation, cell components are separated by difference in mass.
Fractionation is also used for culinary purposes, as coconut oil, palm oil,and palm kernel oils are fractionated to produce oils of different viscosities, that may be used for different purposes. These oils typically use fractional crystallization (separation by solubility at temperatures) for the separation process instead of distillation.
Plasma protein fractionation
Plasma proteins are separated by using the inherent differences of each protein. Fractionation involves changing the conditions of the pooled plasma (e.g., the temperature or the acidity) so that proteins that are normally dissolved in the plasma fluid become insoluble, forming large clumps, called precipitate. The insoluble protein can be collected by centrifugation. One of the very effective ways for carrying out this process is the addition of alcohol to the plasma pool while simultaneously cooling the pool. This process is sometimes called cold alcohol fractionation or ethanol fractionation. It was described by and bears the eponym of Dr Edwin J. Cohn. This procedure is carried out in a series of steps so that a single pool of plasma yields several different protein products, such as albumin and immune globulin.[1][2] Human serum albumin prepared by this process is used in some vaccines, for treating burn victims, and other medical applications.
Fractionation as cancer treatment
Fractionation also refers to a method of treating cancer with radiation therapy. When the total dose of radiation is divided into several, smaller doses over a period of several days, there are fewer toxic effects on healthy cells. This maximizes the effect of radiation on cancer and minimizes the negative side effects. Typical fractionation schemes divide the dose into 30 units delivered every weekday over 6 weeks, though current research is considering the benefits of accelerated fractionation (2 deliveries per day and/or deliveries on weekends as well).
References
- ^ Blood Plasma Pooling (from the Bloodbook.com website)
- ^ Statement by Dr. Kathryn Zoon, Food and Drug Administration (before the U.S. Congress, July 31, 1997
Categories:- Chemical processes
- Laboratory techniques
- Unit operations
Wikimedia Foundation. 2010.
