- Caffeine
-
Caffeine 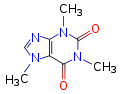

Systematic (IUPAC) name 1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dioneClinical data AHFS/Drugs.com monograph Pregnancy cat. B?(AU) B(US) Legal status Unscheduled (AU) GSL (UK) OTC (US) Routes Oral Pharmacokinetic data Bioavailability 99% Protein binding 17% to 36% Metabolism demethylation by CYP1A2 Half-life 5 hrs Excretion urine Identifiers CAS number 58-08-2 ATC code N06BC01 PubChem CID 2519 DrugBank DB00201 ChemSpider 2424 
UNII 3G6A5W338E 
KEGG D00528 
ChEBI CHEBI:27732 
ChEMBL CHEMBL113 
Chemical data Formula C8H10N4O2 Mol. mass 194.19 SMILES eMolecules & PubChem Properties: Molecular formula C8H10N4O2 Molar mass 194.19 g mol−1 Exact mass 194.080376 u Appearance Odorless, white needles or powder Density 1.23 g/cm3, solid[1] Melting point 227–228 °C, 500-501 K (anhydrous)
234–235 °C, 507-508 K (monohydrate)Boiling point 178 °C, 451 K, 352 °F (subl.)
Solubility in water 2.17 g/100 mL (25 °C)
18.0 g/100 mL (80 °C)
67.0 g/100 mL (100 °C)Acidity (pKa) −0.13–1.22[2] Dipole moment 3.64 D (calculated) Hazards: MSDS ICSC 0405 EU Index 613-086-00-5 EU classification Harmful (Xn) R-phrases R22 S-phrases (S2) NFPA 704 LD50 192 mg/kg (rat, oral)[3] Caffeine is a bitter, white crystalline xanthine alkaloid that acts as a stimulant drug. Caffeine is found in varying quantities in the seeds, leaves, and fruit of some plants, where it acts as a natural pesticide that paralyzes and kills certain insects feeding on the plants. It is most commonly consumed by humans in infusions extracted from the bean of the coffee plant and the leaves of the tea bush, as well as from various foods and drinks containing products derived from the kola nut. Other sources include yerba maté, guarana berries, guayusa, and the yaupon holly.
In humans, caffeine acts as a central nervous system stimulant, temporarily warding off drowsiness and restoring alertness. It is the world's most widely consumed psychoactive drug, but, unlike many other psychoactive substances, it is legal and unregulated in nearly all parts of the world. Beverages containing caffeine, such as coffee, tea, soft drinks, and energy drinks, enjoy great popularity; in North America, 90% of adults consume caffeine daily.[4]
Caffeine is toxic at sufficiently high doses, but ordinary consumption poses few known risks to health, even when carried on for years — there may actually be a modest protective effect against some diseases, including certain types of cancer. Some people experience sleep disruption if they consume caffeine, especially during the evening hours, but other people show little disturbance: the effect of caffeine on sleep is highly variable across individuals. Evidence for a risk to pregnancy is equivocal, but some authorities have concluded that prudent advice is for pregnant women to limit consumption to the equivalent of two cups of coffee per day or less. Caffeine has diuretic properties when administered to people who are not used to it, but regular users develop a strong tolerance to this effect, and studies have generally failed to support the common notion that ordinary consumption of caffeinated beverages contributes significantly to dehydration.
Contents
Health effects
Main article: Health effects of caffeineStimulant effects
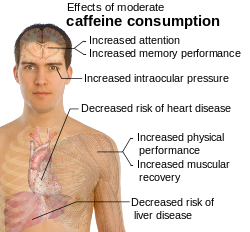
Caffeine is a central nervous system and metabolic stimulant,[6] and is used both recreationally and medically to reduce physical fatigue and to restore alertness when drowsiness occurs. It produces increased wakefulness, faster and clearer flow of thought, increased focus, and better general body coordination.[7] The amount of caffeine necessary to produce effects varies from person to person, depending on body size and degree of tolerance. Effects begin less than an hour after consumption, and a moderate dose usually wears off in about five hours.[7]
Caffeine has a number of effects on sleep, but does not affect all people in the same way. It improves performance during sleep deprivation but may lead to subsequent insomnia.[8] In shift workers it leads to fewer mistakes caused by tiredness.[9] In athletics, moderate doses of caffeine can improve sprint,[10] endurance[11] and team sports performance,[12] but the improvements are not usually very large. High doses of caffeine, however, can impair athletic performance by interfering with coordination.[13] Evidence shows that, contrary to common advice, caffeine may be helpful at high altitude.[14]
Physical effects
Consumption of large amounts of caffeine — usually more than 500 mg per day — especially over extended periods of time, can lead to a condition known as caffeinism.[15] Caffeinism usually combines caffeine dependency with a wide range of unpleasant physical and mental conditions including nervousness, irritability, restlessness, insomnia, headaches, and heart palpitations.[citation needed]
Coffee consumption is associated with a lower overall risk of cancer.[16] This is primarily due to a decrease in the risks of hepatocellular and endometrial cancer, but it may also have a modest effects on colorectal cancer.[17] There does not appear to be a significant protective effect against other types of cancers, and heavy coffee consumption may increase the risk of bladder cancer.[17]
There is little or no evidence that caffeine consumption increases the risk of cardiovascular disease, and it may somewhat reduce the risk of type 2 diabetes.[18] There is some evidence that drinking four or more cups of coffee per day may have a minor protective effect against hypertension; however abstaining from caffeine may reduce the risks even more.[19]
Caffeine increases intraocular pressure in those with glaucoma but does not appear to affect normal individuals.[20] It may protect people from liver cirrhosis.[21] There is no evidence that coffee stunts a child's growth.[22] Caffeine may increase the effectiveness of some medications including ones used to treat headaches.[23]
Caffeine consumption during pregnancy does not appear to increase the risk of congenital malformations, miscarriage or growth retardation even when consumed in moderate to high amounts.[24] However as the data supporting this conclusion is of poor quality some suggest limiting caffeine consumption during pregnancy.[25][26] For example the UK Food Standards Agency has recommended that pregnant women should limit their caffeine intake, out of prudence, to less than 200 mg of caffeine a day—the equivalent of two cups of instant coffee, or one and a half to two cups of fresh coffee.[27] Although the evidence that caffeine may be harmful during pregnancy is equivocal, there is clear evidence that the hormonal changes associated with pregnancy slow the metabolic clearance of caffeine from the system, causing a given dose to have longer-lasting effects (as long as 15 hours in the third trimester).[28]
On the positive side, caffeine is the primary treatment of the breathing disorders apnea of prematurity[29] and may also be effective in preventing bronchopulmonary dysplasia in premature infants.[30] The only short-term risk associated with caffeine citrate treatment is a temporary reduction in weight gain during the therapy,[31] and longer term studies (18 to 21 months) have shown lasting benefits of treatment of premature infants with caffeine.[32] The possibility of subtle long-term developmental problems cannot, however, entirely be ruled out.[33]
When doses of caffeine equivalent to 2-3 cups of coffee are administered to people who have not consumed caffeine over the previous days, they produce a stimulation in urinary output.[34] Because of this diuretic effect, some authorities have recommended that athletes or airline passengers avoid caffeine in order to reduce the risk of dehydration.[34] Most people who consume caffeine, however, ingest it daily. Regular users of caffeine have been shown to develop a strong tolerance to the diuretic effect,[34] and studies have generally failed to support the notion that ordinary consumption of caffeinated beverages contributes significantly to dehydration, even in athletes.[35][36][37]
Psychological
Four caffeine-induced disorders are recognized by the American Psychological Association (APA) including: caffeine intoxication, caffeine-induced sleep disorder, caffeine-induced anxiety disorder and caffeine-related disorder not otherwise specified (NOS).[38] In moderate doses it may reduce symptoms of depression and lower suicide risk.[39] High doses may trigger anxiety and rarely mania and psychosis. As of 2010 the effect of caffeine on people with ADHD is not known.[39] The DSM-IV defines caffeine-induced sleep disorder, as an individual who regularly ingests high doses of caffeine sufficient to induce a significant disturbance in his or her sleep, sufficiently severe to warrant clinical attention.[38]
Caffeine can have both positive and negative effects on anxiety disorders depending on the dose. At high doses, typically greater than 300 mg, it can both cause and worsen anxiety. At low doses it may reduce symptoms of anxiety. Caffeine withdrawal, on the other hand, can cause an increase in anxiety level.[39] In moderate doses caffeine typically does not affect learning or memory.[40] It does however improve cognitive function in people who are fatigued, due to its effect on alertness. Some studies have however found a modest protective against Alzheimer disease, but the evidence is inconclusive.[41]
Caffeine intoxication
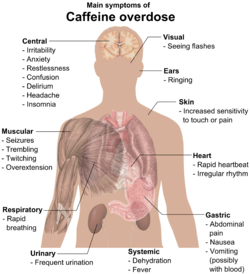
Caffeine overdose can result in a state of central nervous system over-stimulation called caffeine intoxication (DSM-IV 305.90),[38] or colloquially the "caffeine jitters". The symptoms of caffeine intoxication are not unlike overdoses of other stimulants. It may include restlessness, fidgeting, anxiety, excitement, insomnia, flushing of the face, increased urination, gastrointestinal disturbance, muscle twitching, a rambling flow of thought and speech, irritability, irregular or rapid heart beat, and psychomotor agitation.[42] In cases of much larger overdoses, mania, depression, lapses in judgment, disorientation, disinhibition, delusions, hallucinations, or psychosis may occur, and rhabdomyolysis (breakdown of skeletal muscle tissue) can be provoked.[43][44]
Extreme overdose can result in death.[45] The median lethal dose (LD50) given orally, is 192 milligrams per kilogram in rats. The LD50 of caffeine in humans is dependent on weight and individual sensitivity and estimated to be about 150 to 200 milligrams per kilogram of body mass, roughly 80 to 100 cups of coffee for an average adult taken within a limited time frame that is dependent on half-life.[3] Though achieving lethal dose with caffeine would be exceptionally difficult with regular coffee, there have been reported deaths from overdosing on caffeine pills, with serious symptoms of overdose requiring hospitalization occurring from as little as 2 grams of caffeine. An exception to this would be taking a drug such as fluvoxamine or levofloxacin, which blocks the liver enzyme responsible for the metabolism of caffeine, thus increasing the central effects and blood concentrations of caffeine five-fold.[44][45][46] Death typically occurs due to ventricular fibrillation brought about by effects of caffeine on the cardiovascular system.
Treatment of severe caffeine intoxication is generally supportive, providing treatment of the immediate symptoms, but if the patient has very high serum levels of caffeine then peritoneal dialysis, hemodialysis, or hemofiltration may be required.[42]
Tolerance and withdrawal
With repetitive use, the stimulatory effects of caffeine are substantially reduced over time, a phenomenon known as a tolerance. Tolerance develops quickly to some (but not all) effects of caffeine, especially among heavy coffee and energy drink consumers.[47] Some coffee drinkers develop tolerance to its sleep-disrupting effects, but others apparently do not.[28] Withdrawal symptoms—including headache, irritability, inability to concentrate, drowsiness, insomnia, and pain in the stomach, upper body, and joints—may appear within 12 to 24 hours after discontinuation of caffeine intake, peak at roughly 48 hours, and usually last from one to five days.[48]
In other animals
While safe in humans, caffeine is considerably toxic to various animals, such as dogs and birds.[49][50]
The increased toxicity of caffeine in some animals is at least partly due to a poorer ability to metabolize the compound.[51]
Caffeine also has a pronounced effect on mollusks, various insects, and spiders.[52]
Sources and consumption
Global consumption of caffeine has been estimated at 120,000 tonnes per year, making it the world's most popular psychoactive substance. This amounts to one serving of a caffeinated beverage for every person every day.[53]
Caffeine is found in many plant species, where it acts as a natural pesticide, with high caffeine levels being observed in seedlings still developing foliage but lacking mechanical protection;[54] caffeine paralyzes and kills certain insects feeding upon the plant.[55] High caffeine levels have also been found in the surrounding soil of coffee bean seedlings. Therefore, caffeine is understood to have a natural function as both a natural pesticide and an inhibitor of seed germination of other nearby coffee seedlings, thus giving it a better chance of survival.[56]
Caffeine Content in Select Food and Drugs[57][58][59] Product Serving size Caffeine per serving (mg) Caffeine per liter (mg) Caffeine tablet (regular-strength) 1 tablet 100 — Caffeine tablet (extra-strength) 1 tablet 200 — Excedrin tablet 1 tablet 65 — Hershey's Special Dark (45% cacao content) 1 bar (43 g; 1.5 oz) 31 — Hershey's Milk Chocolate (11% cacao content) 1 bar (43 g; 1.5 oz) 10 — Percolated coffee 207 mL (7 U.S. fl oz) 80–135 386–652 Drip coffee 207 mL (7 U.S. fl oz) 115–175 555–845 Coffee, decaffeinated 207 mL (7 U.S. fl oz) 5–15 24–72 Coffee, espresso 44–60 mL (1.5-2 U.S. fl oz) 100 1,691–2254 Black tea 177 mL (6 U.S. fl oz) 50 282 Green tea 177 mL (6 U.S. fl oz) 30 170 Guayakí yerba mate (loose leaf) 6 g (0.2 U.S. oz) 85[60] 358 about Coca-Cola Classic 355 mL (12 U.S. fl oz) 34 96 Mountain Dew 355 mL (12 U.S. fl oz) 54 154 Guaraná Antarctica 350 mL (11 U.S. fl oz) 30 100 Jolt Cola 695 mL (23.5 U.S. fl oz) 280 403 Red Bull 250 mL (8.4 U.S. fl oz) 80 320 Common sources of caffeine are coffee, tea, and (to a lesser extent) chocolate derived from cocoa beans.[61] Less commonly used sources of caffeine include the yerba maté, guarana and ilex guayusa plants,[62] which are sometimes used in the preparation of teas and energy drinks. Two of caffeine's alternative names, mateine and guaranine, are derived from the names of these plants.[63][64] The disparity in experience and effects between the various natural caffeine sources could be because plant sources of caffeine also contain widely varying mixtures of other xanthine alkaloids, including the cardiac stimulants theophylline and theobromine, and other substances such as polyphenols that can form insoluble complexes with caffeine.[65]
One of the world's primary sources of caffeine is the coffee "bean" (which is the seed of the coffee plant), from which coffee is brewed. Caffeine content in coffee varies widely depending on the type of coffee bean and the method of preparation used;[66] even beans within a given bush can show variations in concentration. In general, one serving of coffee ranges from 80–100 milligrams, for a single shot (30 milliliters) of arabica-variety espresso, to approximately 100–125 milligrams for a cup (120 milliliters) of drip coffee. In general, dark-roast coffee has very slightly less caffeine than lighter roasts because the roasting process reduces a small amount of the bean's caffeine content.[67][68] Arabica coffee normally contains significantly (+/-50%) less caffeine than the robusta variety.[66] Tea is another common source of caffeine. Although tea contains more caffeine than coffee (by dry weight), a typical serving contains much less, as tea is normally brewed much weaker. Besides strength of the brew, growing conditions, processing techniques and other variables also affect caffeine content. Certain types of tea may contain somewhat more caffeine than other teas. Tea contains small amounts of theobromine and slightly higher levels of theophylline than coffee. Preparation and many other factors have a significant impact on tea, and color is a very poor indicator of caffeine content. Teas like the pale Japanese green tea, gyokuro, for example, contain far more caffeine than much darker teas like lapsang souchong, which has very little.[69]
Caffeine is also a common ingredient of soft drinks, such as cola, originally prepared from kola nuts. Soft drinks typically contain about 10 to 50 milligrams of caffeine per serving. By contrast, energy drinks, such as Red Bull, can start at 80 milligrams of caffeine per serving. The caffeine in these drinks either originates from the ingredients used or is an additive derived from the product of decaffeination or from chemical synthesis. Guarana, a prime ingredient of energy drinks, contains large amounts of caffeine with small amounts of theobromine and theophylline in a naturally occurring slow-release excipient.[70]
Chocolate derived from cocoa beans contains a small amount of caffeine. The weak stimulant effect of chocolate may be due to a combination of theobromine and theophylline, as well as caffeine.[71] A typical 28-gram serving of a milk chocolate bar has about as much caffeine as a cup of decaffeinated coffee, although some dark chocolate currently in production contains as much as 160 mg per 100g.[58]
Various manufacturers market caffeine tablets, claiming that using caffeine of pharmaceutical quality improves mental alertness. These effects have been borne out by research that shows caffeine use (whether in tablet form or not) results in decreased fatigue and increased attentiveness.[7] These tablets are commonly used by students studying for their exams and by people who work or drive for long hours.[72] One U.S. company is also marketing dissolving caffeine strips as an alternative to energy drinks.[73] Another unusual intake route is SpazzStick, a caffeinated lip balm.[74]
Chemical properties and biosynthesis


Caffeine is an achiral molecule[78] without stereoisomers.[79]
The two amide groups of caffeine exist predominately as zwitterionic resonance structures where the nitrogen and carbon atoms are double bonded to each other so that both of these nitrogen atoms are essentially planar (in sp2 orbital hybridization). The fused ring system therefore contains a total of ten pi electrons and hence according to Hückel's rule is aromatic.[citation needed]
Caffeine is synthesized in plants from the purine nucleotides AMP, GMP, and IMP. These in turn are transformed into xanthosine and then theobromine, the latter being the penultimate precursor of caffeine.[80]
Being readily available as a byproduct of decaffeination, caffeine is not usually synthesized chemically.[81] If desired, it may be synthesized from dimethylurea and malonic acid.[76][77][82]
Pure anhydrous caffeine is a white colorless powder with a melting point of 227–228 °C. Caffeine is moderately soluble in water at room temperature (2 g/100 mL), but very soluble in boiling water (66 g/100 mL). It is also moderately soluble in ethanol (1.5 g/100 mL). It is weakly basic (pKa = ~0.6) requiring strong acid to protonate it.[2]
Pharmacology
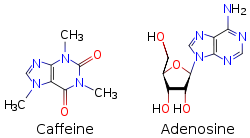
Inside the body caffeine acts through several mechanisms, but its most important effect is to counteract a substance called adenosine that naturally circulates at high levels throughout the body, and especially in the nervous system. In the brain, adenosine plays a generally protective role, part of which is to reduce neural activity levels — for example, there is some evidence that adenosine helps to induce torpor in animals that seasonally hibernate.[83]
Mechanism of action
Because caffeine is both water-soluble and lipid-soluble, it readily crosses the blood–brain barrier that separates the bloodstream from the interior of the brain. Once in the brain, the principal mode of action is as a nonselective antagonist of adenosine receptors (in other words, an agent that reduces the effects of adenosine). The caffeine molecule is structurally similar to adenosine, and is capable of binding to adenosine receptors on the surface of cells without activating them, thereby acting as a competitive inhibitor.[84]
Adenosine is found in every part of the body, because it plays a role in the fundamental ATP-related energy producing mechanism and is also necessary for RNA synthesis, but it has additional functions in the brain. The evidence indicates that brain adenosine acts to protect the brain by suppressing neural activity and by increasing blood flow via receptors located on vascular smooth muscle.[85] Brain adenosine levels are increased by various types of metabolic stress, including lack of oxygen and interruption of blood flow. There is evidence that adenosine functions as a synaptically released neurotransmitter in some parts of the brain; however, stress-related adenosine increases appear to be produced mainly by extracellular metabolism of ATP. Unlike most neurotransmitters, adenosine does not seem to be packaged into vesicles that are released in a voltage-controlled manner, but the possibility of such a mechanism has not entirely been ruled out.[85]
Several classes of adenosine receptors have been described, with different anatomical distributions. A1 receptors are widely distributed, and act to inhibit calcium uptake. A2A receptors are heavily concentrated in the basal ganglia, an area that plays a critical role in behavior control, but can be found in other parts of the brain as well, in lower densities. There is evidence that A 2A receptors interact with the dopamine system, which is involved in reward and arousal. (A2A receptors can also be found on arterial walls and blood cell membranes.)[86]
Beyond its general neuroprotective effects, there are reasons to believe that adenosine may be more specifically involved in control of the sleep-wake cycle. Robert McCarley and his colleagues have argued that accumulation of adenosine may be a primary cause of the sensation of sleepiness that follows prolonged mental activity, and that the effects may be mediated both by inhibition of wake-promoting neurons via A1 receptors, and activation of sleep-promoting neurons via indirect effects on A2A receptors.[86] More recent studies have provided additional evidence for the importance of A2A, but not A1, receptors.[87]
A number of potential mechanisms have been proposed for the athletic performance-enhancing effects of caffeine.[88] In the classic, or metabolic theory, caffeine may increase fat utilization and decrease glycogen utilization. Caffeine mobilizes free fatty acids from fat and/or intramuscular triglycerides by increasing circulating epinephrine levels. The increased availability of free fatty acids increases fat oxidation and spares muscle glycogen, thereby enhancing endurance performance. In the nervous system, caffeine may reduce the perception of effort by lowering the neuron activation threshold, making it easier to recruit the muscles for exercise.[89]
Caffeine metabolites
Metabolites of caffeine also contribute to caffeine's effects. Paraxanthine is responsible for an increase in the lipolysis process, which releases glycerol and fatty acids into the blood to be used as a source of fuel by the muscles. Theobromine is a vasodilator that increases the amount of oxygen and nutrient flow to the brain and muscles. Theophylline acts as a smooth muscle relaxant that chiefly affects bronchioles and acts as a chronotrope and inotrope that increases heart rate and force of contraction.[90]
Metabolism
 Caffeine is metabolized in the liver into three primary metabolites: paraxanthine (84%), theobromine (12%), and theophylline (4%)
Caffeine is metabolized in the liver into three primary metabolites: paraxanthine (84%), theobromine (12%), and theophylline (4%)
Caffeine from coffee or other beverages is absorbed by the small intestine within 45 minutes of ingestion and then distributed throughout all tissues of the body.[91] Peak blood concentration is reached within one hour.[92] It is eliminated by first-order kinetics.[93] Caffeine can also be ingested rectally, evidenced by the formulation of suppositories of ergotamine tartrate and caffeine (for the relief of migraine)[94] and chlorobutanol and caffeine (for the treatment of hyperemesis).[95]
The biological half-life of caffeine—the time required for the body to eliminate one-half of the total amount of caffeine—varies widely among individuals according to such factors as age, liver function, pregnancy, some concurrent medications, and the level of enzymes in the liver needed for caffeine metabolism. It can also be significantly altered by drugs or hormonal states. In healthy adults, caffeine's half-life is approximately 4.9 hours.[96] Heavy cigarette smokers show a decrease in half-life of 30-50%, oral contraceptives can double it, and pregnancy can raise it even more, to as much as 15 hours during the last trimester. In newborn infants the half-life can be 80 hours or more; however it drops very rapidly with age, possibly to less than the adult value by the age of 6 months.[28] The antidepressant Fluvoxamine (Luvox) reduces the clearance of caffeine by more than 90%, and prolongs its elimination half-life more than tenfold; from 4.9 hours to 56 hours.[96]
Caffeine is metabolized in the liver by the cytochrome P450 oxidase enzyme system (to be specific, the 1A2 isozyme) into three metabolic dimethylxanthines,[97] each of which has its own effects on the body:
- Paraxanthine (84%): Has the effect of increasing lipolysis, leading to elevated glycerol and free fatty acid levels in the blood plasma.
- Theobromine (12%): Dilates blood vessels and increases urine volume. Theobromine is also the principal alkaloid in the cocoa bean, and therefore chocolate.
- Theophylline (4%): Relaxes smooth muscles of the bronchi, and is used to treat asthma. The therapeutic dose of theophylline, however, is many times greater than the levels attained from caffeine metabolism.[citation needed]
Each of these metabolites is further metabolized and then excreted in the urine. Caffeine can accumulate in individuals with severe liver disease, increasing its half-life.[98]
Some quinolone antibiotics exert an inhibitory effect on the cytochrome P-450 enzyme CYP1A2, thereby reducing clearance of caffeine and thus increasing blood levels.[99]
A 2011 analysis published by PLoS Genetics reviewed five studies covering more than 47,000 subjects of European descent. Researchers determined that habitual caffeine intake is associated with variations in two genes that regulate how quickly the body processes caffeine. Subjects who had a high-intake mutation of either gene on both chromosomes consumed 40 mg more caffeine per day (equivalent to a can of cola) than people who did not.[100]
Detection in biological fluids
Caffeine can be quantified in blood, plasma, or serum to monitor therapy in neonates, confirm a diagnosis of poisoning, or facilitate a medicolegal death investigation. Plasma caffeine levels are usually in the range of 2–10 mg/L in coffee drinkers, 12–36 mg/L in neonates receiving treatment for apnea, and 40–400 mg/L in victims of acute overdosage. Urinary caffeine concentration is frequently measured in competitive sports programs, for which a level in excess of 15 mg/L is usually considered to represent abuse.[101]
Decaffeination
 Fibrous crystals of purified caffeine. Dark field light microscope image, the image covers an area of approx. 11 by 7 mm.
Fibrous crystals of purified caffeine. Dark field light microscope image, the image covers an area of approx. 11 by 7 mm.
Extraction of caffeine from coffee, to produce decaffeinated coffee and caffeine, is an important industrial process and can be performed using a number of different solvents. Benzene, chloroform, trichloroethylene, and dichloromethane have all been used over the years but for reasons of safety, environmental impact, cost, and flavor, they have been superseded by the following main methods:
- Water extraction: Coffee beans are soaked in water. The water, which contains many other compounds in addition to caffeine and contributes to the flavor of coffee, is then passed through activated charcoal, which removes the caffeine. The water can then be put back with the beans and evaporated dry, leaving decaffeinated coffee with its original flavor. Coffee manufacturers recover the caffeine and resell it for use in soft drinks and over-the-counter caffeine tablets.[102]
- Supercritical carbon dioxide extraction: Supercritical carbon dioxide is an excellent nonpolar solvent for caffeine, and is safer than the organic solvents that are otherwise used. The extraction process is simple: CO2 is forced through the green coffee beans at temperatures above 31.1 °C and pressures above 73 atm. Under these conditions, CO2 is in a "supercritical" state: It has gaslike properties that allow it to penetrate deep into the beans but also liquid-like properties that dissolve 97–99% of the caffeine. The caffeine-laden CO2 is then sprayed with high pressure water to remove the caffeine. The caffeine can then be isolated by charcoal adsorption (as above) or by distillation, recrystallization, or reverse osmosis.[102]
- Extraction by organic solvents: Certain organic solvents such as ethyl acetate present much less health and environmental hazard than previously used chlorinated and aromatic organic solvents. Another method is to use triglyceride oils obtained from spent coffee grounds.[102]
History
Caffeine was first isolated from coffee in 1820 by the German chemist Friedlieb Ferdinand Runge, and then independently in 1821 by French chemists Pierre Robiquet, Pierre Pelletier, and Joseph Caventou. Pelletier coined the word "cafeine" from the French word for coffee (café), and this term became the English word "caffeine".
According to Chinese legend, the Chinese emperor Shennong, reputed to have reigned in about 3000 BCE, accidentally discovered tea when he noted that when certain leaves fell into boiling water, a fragrant and restorative drink resulted.[103] Shennong is also mentioned in Lu Yu's Cha Jing, a famous early work on the subject of tea.[104]
The history of coffee has been recorded as far back as the ninth century. During that time, coffee beans were available only in their place of origin, Ethiopia. Legends trace the discovery of coffee either to a Sufi dervish named Omar, or to a goatherder named Kaldi, who observed goats become elated and sleepless at night after grazing on coffee shrubs and, upon trying the berries the goats had been eating, experienced the same vitality.[105] The earliest literary mention of coffee may be a reference to Bunchum in the works of the 9th-century Persian physician al-Razi.[105]:11 The first reliable record of the use of coffee outside Ethiopia comes from Aden, in 1451.[105]:16 The appreciation of coffee as a beverage in Europe dates from the 17th century. The first coffee house in Venice opened some time in the late 1640s.[105]:127 In Britain, the first coffee house was opened in Oxford in 1650.[105]:41 They soon became popular throughout Western Europe, and played a significant role in social relations in the 17th and 18th centuries.[106]
Use of the kola nut, like the coffee berry and tea leaf, appears to have ancient origins. It is chewed in many West African cultures, individually or in a social setting, to restore vitality and ease hunger pangs. In 1911, kola became the focus of one of the earliest documented health scares, when the US government seized 40 barrels and 20 kegs of Coca-Cola syrup in Chattanooga, Tennessee, alleging the caffeine in its drink was "injurious to health".[107] Although the judge ruled in favor of Coca-Cola, two bills were introduced to the U.S. House of Representatives in 1912 to amend the Pure Food and Drug Act, adding caffeine to the list of "habit-forming" and "deleterious" substances, which must be listed on a product's label.[citation needed]
The earliest evidence of cocoa bean use comes from residue found in an ancient Mayan pot dated to 600 BCE. In the New World, chocolate was consumed in a bitter and spicy drink called xocolatl, often seasoned with vanilla, chile pepper, and achiote. Xocolatl was believed to fight fatigue, a belief probably attributable to the theobromine and caffeine content. Chocolate was an important luxury good throughout pre-Columbian Mesoamerica, and cocoa beans were often used as currency.[citation needed]
Xocolatl was introduced to Europe by the Spaniards, and became a popular beverage by 1700. The Spaniards also introduced the cacao tree into the West Indies and the Philippines. It was used in alchemical processes, where it was known as "black bean".[citation needed]
The leaves and stems of the yaupon holly (Ilex vomitoria) were used by Native Americans to brew a tea called asi or the "black drink".[108] Archaeologists have found evidence of this use stretch back far into antiquity, possibly dating to Late Archaic times.[citation needed]
Discovery
In 1819, the German chemist Friedlieb Ferdinand Runge isolated relatively pure caffeine for the first time; he called it "Kaffebase" (i.e., a base that exists in coffee).[109] In 1821, caffeine was isolated both by French chemist Pierre Jean Robiquet and by another pair of French chemists, Pierre-Joseph Pelletier and Joseph Bienaimé Caventou, according to Swedish chemist Jöns Jacob Berzelius in his yearly journal. Furthermore, Berzelius stated the French chemists had made their discoveries independently of any knowledge of Runge's or each other's work.[110]
Pelletier's article on caffeine was the first to use the term in print (in the French form Caféine).[111] It corroborates Berzelius's account:
Caffeine, noun (feminine). Crystallizable substance discovered in coffee in 1821 by Mr. Robiquet. During the same period – while they were searching for quinine in coffee because coffee is considered by several doctors to be a medicine that reduces fevers and because coffee belongs to the same family as the cinchona [quinine] tree – on their part, Mssrs. Pelletier and Caventou obtained caffeine; but because their research had a different goal and because their research had not been finished, they left priority on this subject to Mr. Robiquet. We do not know why Mr. Robiquet has not published the analysis of coffee which he read to the Pharmacy Society. Its publication would have allowed us to make caffeine better known and give us accurate ideas of coffee's composition ...Robiquet was one of the first to isolate and describe the properties of pure caffeine[112] while Pelletier was the first to perform an elemental analysis.[113]
Berzelius later acknowledged Runge's priority in the extraction of caffeine, stating:[114] "However, at this point, it should not remain unmentioned that Runge (in his Phytochemical Discoveries, 1820, pages 146–147) specified the same method and described caffeine under the name Caffeebase a year earlier than Robiquet, to whom the discovery of this substance is usually attributed, having made the first oral announcement about it at a meeting of the Pharmacy Society in Paris.) According to Runge, he did this at the behest of Johann Wolfgang von Goethe."[115] In 1827, M. Oudry isolated "theine" from tea,[116] but it was later proved by Mulder[117] and by Carl Jobst[118] that theine was the same as caffeine.[115] The structure of caffeine was elucidated near the end of the 19th century by Hermann Emil Fischer, who was also the first to achieve its total synthesis. This was part of the work for which Fischer was awarded the Nobel Prize in 1902.[119]
Religion
Some Seventh-day Adventists, Church of God (Restoration) adherents, and Christian Scientists do not consume caffeine. Some from these religions believe that one is not supposed to consume a non-medical, psychoactive substance, or believe that one is not supposed to consume a substance that is addictive. The Church of Jesus Christ of Latter-day Saints has said the following with regard to caffeinated beverages: "With reference to cola drinks, the Church has never officially taken a position on this matter, but the leaders of the Church have advised, and we do now specifically advise, against the use of any drink containing harmful drugs under circumstances that would result in acquiring the habit. Any beverage that contains ingredients harmful to the body should be avoided."[120]
Gaudiya Vaishnavas generally also abstain from caffeine, as it is alleged to cloud the mind and over-stimulate the senses. To be initiated under a guru, one must have had no caffeine (along with alcohol, nicotine and other drugs) for at least a year.[citation needed]
In Islam the main rule on caffeine is that it is permissible. With regard to the caffeine in coffee, Imam Shihab al-Din said: 'it is halal (lawful) to drink, because all things are halal (lawful) except that which God has made haraam (unlawful)'.[121]
References
- ^ Caffeine, International Occupational Safety and Health Information Centre (CIS)
- ^ a b This is the pKa for protonated caffeine, given as a range of values included in Harry G. Brittain, Richard J. Prankerd (2007). Profiles of Drug Substances, Excipients and Related Methodology, volume 33: Critical Compilation of pKa Values for Pharmaceutical Substances. Academic Press. ISBN 0-12-260833-X. http://books.google.com/?id=D3vBu5Tx4XwC&pg=PT15&lpg=PT15.
- ^ a b Peters, Josef M. (1967). "Factors Affecting Caffeine Toxicity: A Review of the Literature". The Journal of Clinical Pharmacology and the Journal of New Drugs (7): 131–141. http://jcp.sagepub.com/content/7/3/131.extract.
- ^ Lovett, Richard (24 September 2005). "Coffee: The demon drink?". New Scientist (2518). http://www.newscientist.com/article.ns?id=mg18725181.700. Retrieved 2009-08-03.(subscription required)
- ^ References are found on image description page in Wikimedia Commons
- ^ Nehlig, A; Daval, JL; Debry, G (1992). "Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects". Brain Research Reviews 17 (2): 139–70. doi:10.1016/0165-0173(92)90012-B. PMID 1356551.
- ^ a b c Bolton, Sanford (1981). "Caffeine: Psychological Effects, Use and Abuse". Orthomolecular Psychiatry 10 (3): 202–211. http://intraspec.ca/1981-v10n03-p202.pdf.
- ^ Snel J, Lorist MM (2011). "Effects of caffeine on sleep and cognition". Prog. Brain Res. 190: 105–17. doi:10.1016/B978-0-444-53817-8.00006-2. PMID 21531247.
- ^ Ker K, Edwards PJ, Felix LM, Blackhall K, Roberts I (2010). "Caffeine for the prevention of injuries and errors in shift workers". Cochrane Database Syst Rev (5): CD008508. doi:10.1002/14651858.CD008508. PMID 20464765.
- ^ Bishop D (December 2010). "Dietary supplements and team-sport performance". Sports Med 40 (12): 995–1017. doi:10.2165/11536870-000000000-00000. PMID 21058748.
- ^ Conger SA, Warren GL, Hardy MA, Millard-Stafford ML (February 2011). "Does caffeine added to carbohydrate provide additional ergogenic benefit for endurance?". Int J Sport Nutr Exerc Metab 21 (1): 71–84. PMID 21411838.
- ^ Astorino TA, Roberson DW (January 2010). "Efficacy of acute caffeine ingestion for short-term high-intensity exercise performance: a systematic review". J Strength Cond Res 24 (1): 257–65. doi:10.1519/JSC.0b013e3181c1f88a. PMID 19924012.
- ^ Tarnopolsky MA (2010). "Caffeine and creatine use in sport". Ann. Nutr. Metab. 57 Suppl 2: 1–8. doi:10.1159/000322696. PMID 21346331.
- ^ Hackett PH (2010). "Caffeine at high altitude: java at base cAMP". High Alt. Med. Biol. 11 (1): 13–7. doi:10.1089/ham.2009.1077. PMID 20367483.
- ^ Iancu I, Olmer A, Strous RD (2007). "Caffeinism: History, clinical features, diagnosis, and treatment". In Smith BD, Gupta U, Gupta BS. Caffeine and activation theory: effects on health and behavior. CRC Press. pp. 331–344. ISBN 978-0-8493-7102-8.
- ^ Nkondjock, A (2009-05-18). "Coffee consumption and the risk of cancer: an overview.". Cancer letters 277 (2): 121–5. doi:10.1016/j.canlet.2008.08.022. PMID 18834663.
- ^ a b Arab L (2010). "Epidemiologic evidence on coffee and cancer.". Nutrition and cancer 62 (3): 271–83. PMID 20358464.
- ^ van Dam RM (2008). "Coffee consumption and risk of type 2 diabetes, cardiovascular diseases, and cancer.". Applied physiology, nutrition, and metabolism 33 (6): 1269–1283. PMID 19088789.
- ^ Geleijnse JM (2008). "Habitual coffee consumption and blood pressure: an epidemiological perspective". Vasc Health Risk Manag 4: 963–970. PMC 2605331. PMID 19183744. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2605331.
- ^ Li, M; Wang, M, Guo, W, Wang, J, Sun, X (2011 Mar). "The effect of caffeine on intraocular pressure: a systematic review and meta-analysis.". Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 249 (3): 435–42. PMID 20706731.
- ^ Muriel P, Arauz J (July 2010). "Coffee and liver diseases". Fitoterapia 81 (5): 297–305. doi:10.1016/j.fitote.2009.10.003. PMID 19825397.
- ^ O'Connor, Anahad (2007). Never shower in a thunderstorm : surprising facts and misleading myths about our health and the world we live in (1st ed. ed.). New York: Times Books. p. 144. ISBN 978-0-8050-8312-5. http://books.google.com/books?id=neuEbVUZik0C&pg=PA144.
- ^ Gilmore, B; Michael, M (2011-02-01). "Treatment of acute migraine headache.". American family physician 83 (3): 271–80. PMID 21302868.
- ^ Brent RL, Christian MS, Diener RM (April 2011). "Evaluation of the reproductive and developmental risks of caffeine". Birth Defects Res. B Dev. Reprod. Toxicol. 92 (2): 152–87. doi:10.1002/bdrb.20288. PMC 3121964. PMID 21370398. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3121964.
- ^ Kuczkowski KM (November 2009). "Caffeine in pregnancy". Arch. Gynecol. Obstet. 280 (5): 695–8. doi:10.1007/s00404-009-0991-6. PMID 19238414.
- ^ Jahanfar S, Sharifah H (2009). "Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcome". Cochrane Database Syst Rev (2): CD006965. doi:10.1002/14651858.CD006965.pub2. PMID 19370665.
- ^ "Food Standards Agency publishes new caffeine advice for pregnant women". http://www.food.gov.uk/news/pressreleases/2008/nov/caffeineadvice. Retrieved 2009-08-03.
- ^ a b c Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE (March 1999). "Actions of caffeine in the brain with special reference to factors that contribute to its widespread use". Pharmacol. Rev. 51 (1): 83–133. PMID 10049999.
- ^ Mathew OP (May 2011). "Apnea of prematurity: pathogenesis and management strategies". J Perinatol 31 (5): 302–10. doi:10.1038/jp.2010.126. PMID 21127467.
- ^ Kugelman A, Durand M (August 2011). "A comprehensive approach to the prevention of bronchopulmonary dysplasia". Pediatr Pulmonol. doi:10.1002/ppul.21508. PMID 21815280.
- ^ Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W (May 2006). "Caffeine therapy for apnea of prematurity". N. Engl. J. Med. 354 (20): 2112–21. doi:10.1056/NEJMoa054065. PMID 16707748.
- ^ Schmidt B (2005). "Methylxanthine therapy for apnea of prematurity: evaluation of treatment benefits and risks at age 5 years in the international Caffeine for Apnea of Prematurity (CAP) trial". Biol. Neonate 88 (3): 208–13. doi:10.1159/000087584. PMID 16210843.
- ^ Funk GD (November 2009). "Losing sleep over the caffeination of prematurity". J. Physiol. (Lond.) 587 (Pt 22): 5299–300. doi:10.1113/jphysiol.2009.182303. PMC 2793860. PMID 19915211. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2793860.
- ^ a b c Maughan RJ, Griffin J (December 2003). "Caffeine ingestion and fluid balance: a review". J Hum Nutr Diet 16 (6): 411–20. doi:10.1046/j.1365-277X.2003.00477.x. PMID 19774754.
- ^ O'connor, Anahad (2008-03-04). "Really? The claim: caffeine causes dehydration". The New York Times. http://www.nytimes.com/2008/03/04/health/nutrition/04real.html?_r=1. Retrieved 2009-08-03.
- ^ Armstrong LE, Casa DJ, Maresh CM, Ganio MS (July 2007). "Caffeine, fluid-electrolyte balance, temperature regulation, and exercise-heat tolerance". Exerc Sport Sci Rev 35 (3): 135–40. doi:10.1097/jes.0b013e3180a02cc1. PMID 17620932.
- ^ Armstrong LE, Pumerantz AC, Roti MW, Judelson DA, Watson G, Dias JC, Sokmen B, Casa DJ, Maresh CM, Lieberman H, Kellogg M (June 2005). "Fluid, electrolyte, and renal indices of hydration during 11 days of controlled caffeine consumption". Int J Sport Nutr Exerc Metab 15 (3): 252–65. PMID 16131696.
- ^ a b c American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders (4th ed.). American Psychiatric Association. ISBN 0-89042-062-9.
- ^ a b c Lara DR (2010). "Caffeine, mental health, and psychiatric disorders". J. Alzheimers Dis. 20 Suppl 1: S239–48. doi:10.3233/JAD-2010-1378. PMID 20164571.
- ^ Nehlig A (2010). "Is caffeine a cognitive enhancer?". J. Alzheimers Dis. 20 Suppl 1: S85–94. doi:10.3233/JAD-2010-091315. PMID 20182035.
- ^ Santos C, Costa J, Santos J, Vaz-Carneiro A, Lunet N (2010). "Caffeine intake and dementia: systematic review and meta-analysis". J. Alzheimers Dis. 20 Suppl 1: S187–204. doi:10.3233/JAD-2010-091387. PMID 20182026.
- ^ a b c "Caffeine (Systemic)". MedlinePlus. 2000-05-25. Archived from the original on 2007-02-23. http://web.archive.org/web/20070223063601/http://www.nlm.nih.gov/medlineplus/druginfo/uspdi/202105.html. Retrieved 2009-08-03.
- ^ "Caffeine overdose". MedlinePlus. 2006-04-04. http://www.nlm.nih.gov/medlineplus/ency/article/002579.htm. Retrieved 2009-08-03.
- ^ a b Verkhratsky, A. (2005). "Physiology and Pathophysiology of the Calcium Store in the Endoplasmic Reticulum of Neurons". Physiological Reviews 85: 571–2. doi:10.1152/physrev.00004.2004.
- ^ a b Holmgren P, Nordén-Pettersson L, Ahlner J (2004). "Caffeine fatalities—four case reports". Forensic Science International 139 (1): 71–3. PMID 14687776.
- ^ Kerrigan S, Lindsey T (October 2005). "Fatal caffeine overdose: two case reports". Forensic Sci. Int. 153 (1): 67–9. doi:10.1016/j.forsciint.2005.04.016. PMID 15935584.
- ^ http://www.caffeinedependence.org/caffeine_dependence.html
- ^ Juliano LM, Griffiths RR (October 2004). "A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features". Psychopharmacology (Berl.) 176 (1): 1–29. doi:10.1007/s00213-004-2000-x. PMID 15448977.
- ^ Caffeine Poisoning in Dogs | Pet Information
- ^ Why Caffeine is Toxic to Birds | Hotspot for Birds
- ^ Arnaud, MJ (2011). "Pharmacokinetics and metabolism of natural methylxanthines in animal and man". Handbook of Experimental Pharmacology 200: 33–91. PMID 20859793.
- ^ Noever, R., J. Cronise, and R. A. Relwani. 1995. Using spider-web patterns to determine toxicity. NASA Tech Briefs 19(4):82. Published in New Scientist magazine, 29 April 1995
- ^ "What's your poison: caffeine". Australian Broadcasting Corporation. 1997. http://www.abc.net.au/quantum/poison/caffeine/caffeine.htm. Retrieved 2009-08-03.
- ^ Frischknecht, Peter M.; Ulmer-Dufek, Jindra; Baumann, Thomas W. (1986). "Purine alkaloid formation in buds and developing leaflets of Coffea arabica: Expression of an optimal defence strategy?". Phytochemistry 25: 613–6. doi:10.1016/0031-9422(86)88009-8.
- ^ Nathanson JA (October 1984). "Caffeine and related methylxanthines: possible naturally occurring pesticides". Science 226 (4671): 184–7. doi:10.1126/science.6207592. PMID 6207592.
- ^ Baumann, T. W. (1984). "Metabolism and excretion of caffeine during germination of Coffea arabica L". Plant and Cell Physiology 25 (8): 1431–6. http://pcp.oxfordjournals.org/content/25/8/1431.abstract.
- ^ "Caffeine Content of Food and Drugs". Nutrition Action Health Newsletter. Center for Science in the Public Interest. December 1996. Archived from the original on 2007-06-14. http://web.archive.org/web/20070614144016/http://www.cspinet.org/nah/caffeine/caffeine_content.htm. Retrieved 2009-08-03.
- ^ a b "Caffeine Content of Beverages, Foods, & Medications". The Vaults of Erowid. July 7, 2006. http://www.erowid.org/chemicals/caffeine/caffeine_info1.shtml. Retrieved 2009-08-03.
- ^ "Caffeine Content of Drinks". Energy Fiend. http://www.energyfiend.com/the-caffeine-database/. Retrieved 2011-03-04.
- ^ "Traditional Yerba Mate in Biodegradable Bag". Guayaki Yerba Mate. http://guayaki.com/product/41/Traditional-Yerba-Mate-%5B1-lb.%5D.html. Retrieved 2010-07-17.
- ^ Matissek, R (1997). "Evaluation of xanthine derivatives in chocolate: nutritional and chemical aspects". European Food Research and Technology 205 (3): 175–84. http://cat.inist.fr/?aModele=afficheN&cpsidt=2861730.
- ^ "Does Yerba Maté Contain Caffeine or Mateine?". The Vaults of Erowid. December 2003. http://www.erowid.org/plants/yerba_mate/yerba_mate_chemistry1.shtml. Retrieved 2009-08-03.
- ^ "PubChem: mateina". National Library of Medicine. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pccompound&term=mateina. Retrieved 2009-08-03.. Generally translated as mateine in articles written in English
- ^ "PubChem: guaranine". National Library of Medicine. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pccompound&term=guaranine. Retrieved 2009-08-16.
- ^ Balentine D. A., Harbowy M. E. and Graham H. N. (1998). G Spiller. ed. Tea: the Plant and its Manufacture; Chemistry and Consumption of the Beverage.
- ^ a b "Caffeine". International Coffee Organization. http://www.ico.org/caffeine.asp. Retrieved 2009-08-01.
- ^ "Coffee and Caffeine FAQ: Does dark roast coffee have less caffeine than light roast?". http://coffeefaq.com/site/node/15. Retrieved 2009-08-02.
- ^ "All About Coffee: Caffeine Level". Jeremiah's Pick Coffee Co. http://www.jeremiahspick.com/caffeine-e-13.html. Retrieved 2009-08-03.[dead link]
- ^ Hicks, Monique B.; Hsieh, Y-H. Peggy; Bell, Leonard N. (1996). "Tea preparation and its influence on methylxanthine concentration". Food Research International 29: 325–330.
- ^ Bempong DK, Houghton PJ, Steadman K (1993). "The xanthine content of guarana and its preparations". Int J Pharmacog 31 (3): 175–181. doi:10.3109/13880209309082937. ISSN 0925-1618.
- ^ Smit, Hendrik J.; Gaffan, Elizabeth A.; Rogers, Peter J. (2004). "Methylxanthines are the psycho-pharmacologically active constituents of chocolate". Psychopharmacology 176 (3–4): 412–9. doi:10.1007/s00213-004-1898-3. PMID 15549276.
- ^ Bennett Alan Weinberg, Bonnie K. Bealer (2001). The world of caffeine. Routledge. p. 195. ISBN 0-415-92722-6. http://books.google.com/?id=YdpL2YCGLVYC&pg=PA195.
- ^ LeBron James Shills for Sheets Caffeine Strips, a Bad Idea for Teens, Experts Say - ABC News
- ^ Nancy Shute Over The Limit:Americans young and old crave high-octane fuel, and doctors are jittery US News and World Reports. 15 APR 07. Accessed 10/18/11
- ^ "caffeine biosynthesis". The Enzyme Database. Trinity College Dublin. http://www.enzyme-database.org/reaction/misc/caffeine.html. Retrieved 2011-09-24.
- ^ a b Temple NJ, Wilson T (2003). Beverages in Nutrition and Health. Totowa, NJ: Humana Press. p. 172. ISBN 1-58829-173-1.
- ^ a b US patent 2785162, Swidinsky J, Baizer MM, "Process for the formylation of a 5-nitrouracil", published 1957-03-12, assigned to New York Quinine and Chemical Works, Inc.
- ^ Vallombroso T (2001). Organic Chemistry Pearls of Wisdom. Boston Medical Publishing Corp. p. 43. ISBN 1-58409-016-2.
- ^ Klosterman L (2006). The Facts About Caffeine (Drugs). Benchmark Books (NY). p. 43. ISBN 0-7614-2242-0.
- ^ Ashihara H, Monteiro AM, Gillies FM, Crozier A (July 1996). "Biosynthesis of Caffeine in Leaves of Coffee". Plant Physiol. 111 (3): 747–753. doi:10.1104/pp.111.3.747. PMC 157891. PMID 12226327. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=157891.
- ^ Simon Tilling. "Crystalline Caffeine". Bristol University. http://www.chm.bris.ac.uk/webprojects2001/tilling/synthesis.htm. Retrieved 2009-08-03.
- ^ Zajac MA, Zakrzewski AG, Kowal MG, Narayan S (2003). "A Novel Method of Caffeine Synthesis from Uracil". Synthetic Communications 33 (19): 3291–3297. doi:10.1081/SCC-120023986. http://www.umich.edu/~chemh215/CHEM216/Honors%20Cup_old/HCProposal/caffeine.pdf.
- ^ Jinka TR, Tøien Ø, Drew KL (July 2011). "Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A(1) receptors". J. Neurosci. 31 (30): 10752–8. doi:10.1523/JNEUROSCI.1240-11.2011. PMID 21795527.
- ^ Fisone G, Borgkvist A, Usiello A (April 2004). "Caffeine as a psychomotor stimulant: mechanism of action". Cell. Mol. Life Sci. 61 (7-8): 857–72. doi:10.1007/s00018-003-3269-3. PMID 15095008.
- ^ a b Latini S, Pedata F (November 2001). "Adenosine in the central nervous system: release mechanisms and extracellular concentrations". J. Neurochem. 79 (3): 463–84. doi:10.1046/j.1471-4159.2001.00607.x. PMID 11701750.
- ^ a b Basheer R, Strecker RE, Thakkar MM, McCarley RW (August 2004). "Adenosine and sleep-wake regulation". Prog. Neurobiol. 73 (6): 379–96. doi:10.1016/j.pneurobio.2004.06.004. PMID 15313333.
- ^ Huang ZL, Qu WM, Eguchi N, et al. (July 2005). "Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine". Nat. Neurosci. 8 (7): 858–9. doi:10.1038/nn1491. PMID 15965471.
- ^ Davis, JK; Green, JM (2009). "Caffeine and anaerobic performance: ergogenic value and mechanisms of action.". Sports medicine (Auckland, N.Z.) 39 (10): 813–32. PMID 19757860.
- ^ McArdle, William (2010). Exercise Physiology. 7th edition. Baltimore, MD: Lippincott Williams and Wilkins. p. 559. ISBN 978-0-7817-9781-8.
- ^ Dews, P.B. (1984). Caffeine: Perspectives from Recent Research. Berlin: Springer-Valerag. ISBN 978-0-387-13532-8.
- ^ Liguori A, Hughes JR, Grass JA (November 1997). "Absorption and subjective effects of caffeine from coffee, cola and capsules". Pharmacol. Biochem. Behav. 58 (3): 721–6. doi:10.1016/S0091-3057(97)00003-8. PMID 9329065.
- ^ Caffeine component of Koffazon], taken from Fass.se (Swedish Drug Catalog). Last updated 2010-02-10
- ^ Newton R, Broughton LJ, Lind MJ, Morrison PJ, Rogers HJ, Bradbrook ID (1981). "Plasma and salivary pharmacokinetics of caffeine in man". Eur. J. Clin. Pharmacol. 21 (1): 45–52. doi:10.1007/BF00609587. PMID 7333346.
- ^ Graham JR (June 1954). "Rectal use of ergotamine tartrate and caffeine alkaloid for the relief of migraine". N. Engl. J. Med. 250 (22): 936–8. doi:10.1056/NEJM195406032502203. PMID 13165929.
- ^ Brødbaek HB, Damkier P (May 2007). "[The treatment of hyperemesis gravidarum with chlorobutanol-caffeine rectal suppositories in Denmark: practice and evidence]" (in Danish). Ugeskr. Laeg. 169 (22): 2122–3. PMID 17553397.
- ^ a b "Drug Interaction: Caffeine Oral and Fluvoxamine Oral". Medscape Multi-Drug Interaction Checker. http://www.medscape.com/druginfo/druginteractions?drug_408=Caffeine%20Oral&drug_1049=Fluvoxamine%20Oral.
- ^ "Caffeine". The Pharmacogenetics and Pharmacogenomics Knowledge Base. http://www.pharmgkb.org/do/serve?objId=PA448710&objCls=Drug&tabType=Properties#biotransformation. Retrieved 2010-10-25.
- ^ Verbeeck RK (December 2008). "Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction". Eur. J. Clin. Pharmacol. 64 (12): 1147–61. doi:10.1007/s00228-008-0553-z. PMID 18762933.
- ^ Janknegt R (November 1990). "Drug interactions with quinolones". J. Antimicrob. Chemother. 26 Suppl D: 7–29. PMID 2286594.
- ^ Cornelis MC, Monda KL, Yu K, et al. (April 2011). "Genome-wide meta-analysis identifies regions on 7p21 (AHR) and 15q24 (CYP1A2) as determinants of habitual caffeine consumption". PLoS Genet. 7 (4): e1002033. doi:10.1371/journal.pgen.1002033. PMC 3071630. PMID 21490707. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3071630.
- ^ Baselt, R. (2011). Disposition of Toxic Drugs and Chemicals in Man (9th ed.). Seal Beach, CA: Biomedical Publications. pp. 236–9. ISBN 0-931890-08-X.
- ^ a b c Senese, Fred (2005-09-20). "How is coffee decaffeinated?". General Chemistry Online. http://antoine.frostburg.edu/chem/senese/101/consumer/faq/decaffeinating-coffee.shtml. Retrieved 2009-08-03.
- ^ John C. Evans (1992). Tea in China: The History of China's National Drink. Greenwood Press. p. 2. ISBN 0-313-28049-5.
- ^ Yu, Lu (1995). The Classic of Tea: Origins & Rituals. Ecco Pr. ISBN 0-88001-416-4.
- ^ a b c d e Ukers WH (1922). All About Coffee. New York: The Tea and Coffee Trade Journal Company. pp. 13–15. ISBN 0-8103-4092-5. http://books.google.com/?id=Y5tXt7aoLNoC.
- ^ "Coffee". Encyclopædia Britannica. 1911.
- ^ Benjamin LT, Rogers AM, Rosenbaum A (January 1991). "Coca-Cola, caffeine, and mental deficiency: Harry Hollingworth and the Chattanooga trial of 1911". J Hist Behav Sci 27 (1): 42–55. doi:10.1002/1520-6696(199101)27:1<42::AID-JHBS2300270105>3.0.CO;2-1. PMID 2010614.
- ^ Fairbanks, Charles H. (2004). "The function of black drink among the Creeks". In Hudson, Charles M.. Black Drink. University of Georgia Press. p. 123. ISBN 978-0-8203-2696-2.
- ^ Runge, Friedlieb Ferdinand (1820). Neueste phytochemische Entdeckungen zur Begründung einer wissenschaftlichen Phytochemie [Latest phytochemical discoveries for the founding of a scientific phytochemistry]. Berlin: G. Reimer. pp. 144–159. http://books.google.com/books?id=KLg5AAAAcAAJ&pg=P146.
- ^ "[Annual report on the progress of the physical sciences by Jacob Berzelius]" (in German) Jahres-Bericht über die Fortschritte der physischen Wissenschaften von Jacob Berzelius. 4. 1825. p. 180. http://books.google.com/books?id=XJI8AAAAIAAJ&pg=RA1-PA180.
- ^ Pelletier, Pierre Joseph (April 1822). "Cafeine" (in French). Dictionnaire de Médecine. 4. Paris: Béchet Jeune. pp. 35–36. http://books.google.com/books?id=rFw_AAAAcAAJ&pg=PA35. Retrieved 2011-03-03.
- ^ Robiquet, Pierre Jean (1823). "Cafe" (in French). Dictionnaire Technologique, ou Nouveau Dictionnaire Universel des Arts et Métiers. 4. Paris: Thomine et Fortic. pp. 50–61. http://cnum.cnam.fr/CGI/gpage.cgi?p1=50. Retrieved 2011-03-03.
- ^ Dumas and Pelletier (1823). "Recherches sur la composition élémentaire et sur quelques propriétés caractéristiques des bases salifiables organiques [Studies into the elemental composition and some characteristic properties of organic bases]" (in French). Annales de Chimie et de Physique 24: 163–191. http://books.google.com/books?id=-BIAAAAAMAAJ&pg=PA182.
- ^ (in German) Jahres-Bericht über die Fortschritte der physischen Wissenschaften von Jacob Berzelius. 7. 1828. p. 270. http://books.google.com/books?id=iGs1AAAAcAAJ&pg=P270.
- ^ a b Weinberg BA, Bealer BK (2001). The World of Caffeine. Routledge. ISBN 0-415-92722-6.
- ^ Oudry M (1827). "Note sur la Theine" (in French). Nouvelle bibliothèque médicale 1: 477–479. http://books.google.com/books?id=cGpEAAAAcAAJ&pg=PA477.
- ^ Mulder JM (1838). "Über Kaffein und Thein [Concerning coffee and tea]". Journal für praktische Chemie 15: 280–284.
- ^ Jobst C (1838). "Thein identisch mit Caffein [Theine is identical to caffeine)]". Liebig's Annalen der Chemie und Pharmacie 25: 63–66.
- ^ Hj. Théel (1902). "Nobel Prize Presentation Speech". http://nobelprize.org/nobel_prizes/chemistry/laureates/1902/press.html.
- ^ Doctrine and Covenants Student Manual: Religion 324 and 325. Salt Lake City: LDS Church. 2001. p. 209. http://www.ldsces.org/inst_manuals/dc-in/manualindex.asp.
- ^ Imam Shihab al-Din. "Drinking drinks with caffeine". http://islamweb.net/ver2/Fatwa/ShowFatwa.php?lang=E&Id=84417&Option=FatwaId. Retrieved 2009-08-03.
Stimulants (N06B) Adamantanes Adaphenoxate • Adapromine • Amantadine • Bromantane • Chlodantane • Gludantane • Memantine • Midantane
Adenosine antagonists 8-Chlorotheophylline • 8-Cyclopentyltheophylline • 8-Phenyltheophylline • Aminophylline • Caffeine • CGS-15943 • Dimethazan • Paraxanthine • SCH-58261 • Theobromine • TheophyllineAlkylamines Arylcyclohexylamines Benocyclidine • Dieticyclidine • Esketamine • Eticyclidine • Gacyclidine • Ketamine • Phencyclamine • Phencyclidine • Rolicyclidine • Tenocyclidine • Tiletamine
Benzazepines 6-Br-APB • SKF-77434 • SKF-81297 • SKF-82958
Cholinergics A-84543 • A-366,833 • ABT-202 • ABT-418 • AR-R17779 • Altinicline • Anabasine • Arecoline • Cotinine • Cytisine • Dianicline • Epibatidine • Epiboxidine • GTS-21 • Ispronicline • Nicotine • PHA-543,613 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • SIB-1553A • SSR-180,711 • TC-1698 • TC-1827 • TC-2216 • TC-5619 • Tebanicline • UB-165 • Varenicline • WAY-317,538
Convulsants Anatoxin-a • Bicuculline • DMCM • Flurothyl • Gabazine • Pentetrazol • Picrotoxin • Strychnine • Thujone
Eugeroics Adrafinil • Armodafinil • CRL-40941 • Modafinil
Oxazolines 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone
Phenethylamines 1-(4-Methylphenyl)-2-aminobutane • 1-Phenyl-2-(piperidin-1-yl)pentan-3-one • 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane • 2-Fluoroamphetamine • 2-Fluoromethamphetamine • 2-OH-PEA • 2-Phenyl-3-aminobutane • 2-Phenyl-3-methylaminobutane • 2,3-MDA • 3-Fluoroamphetamine • 3-Fluoroethamphetamine • 3-Fluoromethcathinone • 3-Methoxyamphetamine • 3-Methylamphetamine • 3,4-DMMC • 4-BMC • 4-Ethylamphetamine • 4-FA • 4-FMA • 4-MA • 4-MMA • 4-MTA • 6-FNE • Alfetamine • α-Ethylphenethylamine • Amfecloral • Amfepentorex • Amfepramone • Amidephrine • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Arbutamine • β-Methylphenethylamine • β-Phenylmethamphetamine • Benfluorex • Benzedrone • Benzphetamine • BDB (J) • BOH (Hydroxy-J) • BPAP • Buphedrone • Bupropion (Amfebutamone) • Butylone • Cathine • Cathinone • Chlorphentermine • Cinnamedrine • Clenbuterol • Clobenzorex • Cloforex • Clortermine • D-Deprenyl • Denopamine • Dimethoxyamphetamine • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Dobutamine • DOPA (Dextrodopa, Levodopa) • Dopamine • Dopexamine • Droxidopa • EBDB (Ethyl-J) • Ephedrine • Epinephrine (Adrenaline) • Epinine (Deoxyepinephrine) • Etafedrine • Ethcathinone (Ethylpropion) • Ethylamphetamine (Etilamfetamine) • Ethylnorepinephrine (Butanefrine) • Ethylone • Etilefrine • Famprofazone • Fenbutrazate • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenmetramide • Fenproporex • Flephedrone • Fludorex • Furfenorex • Gepefrine • HMMA • Hordenine • Ibopamine • IMP • Indanylamphetamine • Isoetarine • Isoethcathinone • Isoprenaline (Isoproterenol) • L-Deprenyl (Selegiline) • Lefetamine • Lisdexamfetamine • Lophophine (Homomyristicylamine) • Manifaxine • MBDB (Methyl-J; "Eden") • MDA (Tenamfetamine) • MDBU • MDEA ("Eve") • MDMA ("Ecstasy", "Adam") • MDMPEA (Homarylamine) • MDOH • MDPR • MDPEA (Homopiperonylamine) • Mefenorex • Mephedrone • Mephentermine • Metanephrine • Metaraminol • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methoxamine • Methoxyphenamine • MMA • Methcathinone (Methylpropion) • Methedrone • Methoxyphenamine • Methylone • MMDA • MMDMA • MMMA • Morazone • N-Benzyl-1-phenethylamine • N,N-Dimethylphenethylamine • Naphthylamphetamine • Nisoxetine • Norepinephrine (Noradrenaline) • Norfenefrine • Norfenfluramine • Normetanephrine • Octopamine • Orciprenaline • Ortetamine • Oxilofrine • Paredrine (Norpholedrine, Oxamphetamine, Mycadrine) • PBA • PCA • PHA • Pargyline • Pentorex (Phenpentermine) • Pentylone • Phendimetrazine • Phenmetrazine • Phenpromethamine • Phentermine • Phenylalanine • Phenylephrine (Neosynephrine) • Phenylpropanolamine • Pholedrine • PIA • PMA • PMEA • PMMA • PPAP • Prenylamine • Propylamphetamine • Pseudoephedrine • Radafaxine • Ropinirole • Salbutamol (Albuterol; Levosalbutamol) • Sibutramine • Synephrine (Oxedrine) • Theodrenaline • Tiflorex (Flutiorex) • Tranylcypromine • Tyramine • Tyrosine • Xamoterol • Xylopropamine • Zylofuramine
Piperazines Piperidines 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine • 1-(3,4-Dichlorophenyl)-1-(piperidin-2-yl)butane • 2-Benzylpiperidine • 2-Methyl-3-phenylpiperidine • 3,4-Dichloromethylphenidate • 4-Benzylpiperidine • 4-Methylmethylphenidate • Desoxypipradrol • Difemetorex • Diphenylpyraline • Ethylphenidate • Methylnaphthidate • Methylphenidate (Dexmethylphenidate) • N-Methyl-3β-propyl-4β-(4-chlorophenyl)piperidine • Nocaine • Phacetoperane • Pipradrol • SCH-5472
Pyrrolidines 2-Diphenylmethylpyrrolidine • α-PPP • α-PBP • α-PVP • Diphenylprolinol • MDPPP • MDPBP • MDPV • MPBP • MPHP • MPPP • MOPPP • Naphyrone • PEP • Prolintane • Pyrovalerone
Tropanes 3-CPMT • 3'-Chloro-3α-(diphenylmethoxy)tropane • 3-Pseudotropyl-4-fluorobenzoate • 4'-Fluorococaine • AHN-1055 • Altropane (IACFT) • Brasofensine • CFT (WIN 35,428) • β-CIT (RTI-55) • Cocaethylene • Cocaine • Dichloropane (RTI-111) • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Norcocaine • PIT • PTT • RTI-31 • RTI-32 • RTI-51 • RTI-105 • RTI-112 • RTI-113 • RTI-117 • RTI-120 • RTI-121 (IPCIT) • RTI-126 • RTI-150 • RTI-154 • RTI-171 • RTI-177 • RTI-183 • RTI-193 • RTI-194 • RTI-199 • RTI-202 • RTI-204 • RTI-229 • RTI-241 • RTI-336 • RTI-354 • RTI-371 • RTI-386 • Salicylmethylecgonine • Tesofensine • Troparil (β-CPT, WIN 35,065-2) • Tropoxane • WF-23 • WF-33 • WF-60
Others 1-(Thiophen-2-yl)-2-aminopropane • 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-MDP • 2-Phenylcyclohexylamine • 2-Phenyl-3,6-dimethylmorpholine • 3-Benzhydrylmorpholine • 3,3-Diphenylcyclobutanamine • 5-(2-Aminopropyl)indole • 5-Iodo-2-aminoindane • AL-1095 • Amfonelic acid • Amineptine • Amiphenazole • Atipamezole • Atomoxetine (Tomoxetine) • Bemegride • Benzydamine • BTQ • BTS 74,398 • Carphedon • Ciclazindol • Cilobamine • Clofenciclan • Cropropamide • Crotetamide • Cypenamine • D-161 • Diclofensine • Dimethocaine • Efaroxan • Etamivan • EXP-561 • Fencamfamine • Fenpentadiol • Feprosidnine • G-130 • Gamfexine • Gilutensin • GSK1360707F • GYKI-52895 • Hexacyclonate • Idazoxan • Indanorex • Indatraline • JNJ-7925476 • JZ-IV-10 • Lazabemide • Leptacline • Levopropylhexedrine • Lomevactone • LR-5182 • Mazindol • Meclofenoxate • Medifoxamine • Mefexamide • Mesocarb • Methastyridone • Methiopropamine • N-Methyl-3-phenylnorbornan-2-amine • Nefopam • Nikethamide • Nomifensine • O-2172 • Oxaprotiline • Phthalimidopropiophenone • PNU-99,194 • Propylhexedrine • PRC200-SS • Rasagiline • Rauwolscine • Rubidium chloride • Setazindol • Tametraline • Tandamine • Trazium • UH-232 • Yohimbine
See also Sympathomimetic amines Psychostimulants, agents used for ADHD, and nootropics (N06B) Centrally acting sympathomimetics Xanthine derivatives Caffeine • FenethyllineGlutamate receptor CX-516 • CX-546 • CX-614 • CX-691 • CX-717 • IDRA-21 • LY-404,187 • LY-503,430 • PEPA • S-18986 • Sunifiram • UnifiramEugeroics / Benzhydryl compounds Histamine H3 receptor antagonists GABAA α5 inverse agonists Dopamine D1 receptor agonists α7 nicotinic agonists / PAMs AR-R17779 • PNU-282,987 • SSR-180,711Prolyl endopeptidase inhibitors S-17092Alpha-adrenergic agonists Other psychostimulants and nootropics Acetylcarnitine • Adafenoxate • Bifemelane • Carbenoxolone • Citicoline • Cyprodenate • Ensaculin • Idebenone • Ispronicline • Deanol • Dimebon • Fipexide • Leteprinim • Linopirdine • Meclofenoxate • Nizofenone • P7C3 • Pirisudanol • Pyritinol • Rubidium • Sulbutiamine • Taltirelin • Tricyanoaminopropene • VinpocetineAdenosinergics Receptor ligands 2-(1-Hexynyl)-N-methyladenosine • 2-Cl-IB-MECA • 2'-MeCCPA • 5'-N-ethylcarboxamidoadenosine • ATL-146e • BAY 60–6583 • CCPA • CGS-21680 • CP-532,903 • GR 79236 • LUF-5835 • LUF-5845 • N6-Cyclopentyladenosine • Regadenoson • SDZ WAG 994 • UK-432,0978-Phenyl-1,3-dipropylxanthine • Acefylline • Aminophylline • Bamifylline • Caffeine • CGS-15943 • 8-Chlorotheophylline • CPX • CVT-6883 • Dimethazan • DPCPX • Fenethylline • Istradefylline • KF-26777 • MRE3008F20 • MRS-1220 • MRS-1334 • MRS-1706 • MRS-1754 • MRS-3777 • Paraxanthine • Pentoxifylline • Preladenant • Propentofylline • PSB-10 • PSB-11 • PSB 36 • PSB-603 • PSB-788 • PSB-1115 • Rolofylline • SCH-442,416 • SCH-58261 • Theobromine • Theophylline • VUF-5574 • ZM-241,385Reuptake inhibitors ENT inhibitorsVNT inhibitorsExternal links
- The Consumers Union Report on Licit and Illicit Drugs, Caffeine-Part 1 Part 2
- Caffeine: ChemSub Online
- Mayo Clinic staff (October 3, 2009). "Caffeine content for coffee, tea, soda and more". Mayo Clinic. http://www.mayoclinic.com/health/caffeine/AN01211. Retrieved 2010-11-08.
- eMedicine Caffeine-Related Psychiatric Disorders
Categories:- Adenosine antagonists
- Caffeine
- Coffee chemistry
- Xanthines
- Anxiogenics
- Bitter compounds
- IARC Group 3 carcinogens
- Phosphodiesterase inhibitors
- Plant toxin insecticides
Wikimedia Foundation. 2010.