- N6-Cyclopentyladenosine
-
N6-Cyclopentyladenosine 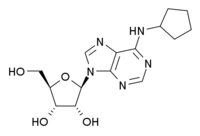 (2R,3R,4S,5R)-2-[6-(cyclopentylamino)purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol
(2R,3R,4S,5R)-2-[6-(cyclopentylamino)purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diolIdentifiers CAS number 41552-82-3 PubChem 657378 Jmol-3D images Image 1 - C1CCC(C1)NC2=NC=NC3=C2N=CN3[C@H]4[C@@H]([C@@H]([C@H](O4)CO)O)O
Properties Molecular formula C15H21N5O4  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references N6-Cyclopentyladenosine (CPA) is a drug which acts as a selective adenosine A1 receptor agonist.[1] It has mainly cardiovascular effects with only subtle alterations of behaviour.[2] CPA is widely used in scientific research into the adenosine receptors and has been used to derive a large family of derivatives.[3][4][5][6][7]
See also
References
- ^ Williams M, Braunwalder A, Erickson TJ (February 1986). "Evaluation of the binding of the A-1 selective adenosine radioligand, cyclopentyladenosine (CPA), to rat brain tissue". Naunyn-Schmiedeberg's Archives of Pharmacology 332 (2): 179–83. doi:10.1007/BF00511410. PMID 3703020.
- ^ Coffin VL, Spealman RD (April 1987). "Behavioral and cardiovascular effects of analogs of adenosine in cynomolgus monkeys". The Journal of Pharmacology and Experimental Therapeutics 241 (1): 76–83. PMID 3572798.
- ^ Zablocki JA, Wu L, Shryock J, Belardinelli L (2004). "Partial A(1) adenosine receptor agonists from a molecular perspective and their potential use as chronic ventricular rate control agents during atrial fibrillation (AF)". Current Topics in Medicinal Chemistry 4 (8): 839–54. doi:10.2174/1568026043450998. PMID 15078215.
- ^ Hutchinson SA, Scammells PJ (2004). "A(1) adenosine receptor agonists: medicinal chemistry and therapeutic potential". Current Pharmaceutical Design 10 (17): 2021–39. doi:10.2174/1381612043384204. PMID 15279543.
- ^ Elzein E, Kalla R, Li X, Perry T, Marquart T, Micklatcher M, Li Y, Wu Y, Zeng D, Zablocki J (January 2007). "N6-Cycloalkyl-2-substituted adenosine derivatives as selective, high affinity adenosine A1 receptor agonists". Bioorganic & Medicinal Chemistry Letters 17 (1): 161–6. doi:10.1016/j.bmcl.2006.09.065. PMID 17045477.
- ^ Elzein E, Zablocki J (December 2008). "A1 adenosine receptor agonists and their potential therapeutic applications". Expert Opinion on Investigational Drugs 17 (12): 1901–10. doi:10.1517/13543780802497284. PMID 19012505.
- ^ Franchetti P, Cappellacci L, Vita P, Petrelli R, Lavecchia A, Kachler S, Klotz KN, Marabese I, Luongo L, Maione S, Grifantini M (April 2009). "N6-Cycloalkyl- and N6-bicycloalkyl-C5'(C2')-modified adenosine derivatives as high-affinity and selective agonists at the human A1 adenosine receptor with antinociceptive effects in mice". Journal of Medicinal Chemistry 52 (8): 2393–406. doi:10.1021/jm801456g. PMID 19317449.
Adenosinergics Receptor ligands 2-(1-Hexynyl)-N-methyladenosine • 2-Cl-IB-MECA • 2'-MeCCPA • 5'-N-ethylcarboxamidoadenosine • ATL-146e • BAY 60–6583 • CCPA • CGS-21680 • CP-532,903 • GR 79236 • LUF-5835 • LUF-5845 • N6-Cyclopentyladenosine • Regadenoson • SDZ WAG 994 • UK-432,0978-Phenyl-1,3-dipropylxanthine • Acefylline • Aminophylline • Bamifylline • Caffeine • CGS-15943 • 8-Chlorotheophylline • CPX • CVT-6883 • Dimethazan • DPCPX • Fenethylline • Istradefylline • KF-26777 • MRE3008F20 • MRS-1220 • MRS-1334 • MRS-1706 • MRS-1754 • MRS-3777 • Paraxanthine • Pentoxifylline • Preladenant • Propentofylline • PSB-10 • PSB-11 • PSB 36 • PSB-603 • PSB-788 • PSB-1115 • Rolofylline • SCH-442,416 • SCH-58261 • Theobromine • Theophylline • VUF-5574 • ZM-241,385Reuptake inhibitors ENT inhibitorsVNT inhibitors
This drug article relating to the cardiovascular system is a stub. You can help Wikipedia by expanding it.
