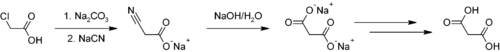- Malonic acid
-
Malonic acid 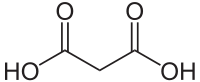
 propanedioic acidOther namesmethanedicarboxylic acid
propanedioic acidOther namesmethanedicarboxylic acidIdentifiers CAS number 141-82-2 
PubChem 867 DrugBank DB02175 ChEMBL CHEMBL7942 
Jmol-3D images Image 1 - O=C(O)CC(O)=O
Properties Molecular formula C3H4O4 Molar mass 104.06 g mol−1 Exact mass 104.010959 Density 1.619 g/cm3 Melting point 135–136 °C
Boiling point decomposes
Solubility in water Miscible Acidity (pKa) pKa1=2.83
pKa2=5.69Related compounds Other anions malonate Related carboxylic acids acetic acid
oxalic acid
propionic acid
tartronic acid
acrylic acid
butyric acid
succinic acid
fumaric acidRelated compounds propanone
propionaldehyde
propanedial
dimethyl malonateHazards MSDS External MSDS  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
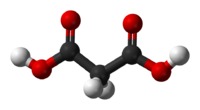
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionised form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's ethyl ester. The name originates from the Greek word μᾶλον (malon) meaning 'apple'.
Contents
Biochemistry
The calcium salt of malonic acid occurs in high concentrations in beetroot. It exists in its normal state as white crystals. Malonic acid is the classic example of a competitive inhibitor: It acts against succinate dehydrogenase (complex II) in the respiratory electron transport chain.
Preparation
A classical preparation of malonic acid starts from chloroacetic acid:[1]
Sodium carbonate generates the sodium salt, which is then reacted with sodium cyanide to provide the cyano acetic acid salt via a nucleophilic substitution. The nitrile group can be hydrolyzed with sodium hydroxide to sodium malonate, and acidification affords malonic acid.
Organic reactions
In a well-known reaction, malonic acid condenses with urea to form barbituric acid. Malonic acid is also frequently used as an enolate in Knoevenagel condensations or condensed with acetone to form Meldrum's acid. The esters of malonic acid are also used as a -CH2COOH synthon in the malonic ester synthesis.
References
- ^ Nathan Weiner, "Malonic acid", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv2p0376; Coll. Vol. 2: 376
External links
Categories:- Malonates
- Dicarboxylic acids
Wikimedia Foundation. 2010.