- Dicarboxylic acid
-
Dicarboxylic acids are organic compounds that contain two carboxylic acid functional groups. In molecular formulae for dicarboxylic acids, these groups are often written as HOOC-R-COOH, where R may be an alkyl, alkenyl, alkynyl, or aryl group. Dicarboxylic acids can be used to prepare copolymers such as polyamides and polyesters.
In general, dicarboxylic acids show the same chemical behaviour and reactivity as monocarboxylic acids. The ionization of the second carboxyl group occurs less readily than the first one. This is because more energy is required to separate a positive hydrogen ion from the anion than from the neutral molecule.
A mnemonic to aid in remembering the order of the common nomenclature for the first six dicarboxylic acids is "Oh my, such great apple pie!" (oxalic, malonic, succinic, glutaric, adipic, pimelic). A variant adds "Sweet as sugar!" (suberic, azelaic, sebacic) to the end of the mnemonic. An additional way of remembering the first six dicarboxylic acids is by simply recalling the acronym OMSGAP, which is a simplification of the previously described mnemonic device.
When one of the carboxy groups is replaced with an aldehyde group, the resulting structure is called a "aldehydic acid".
Contents
Examples
Elementary saturated dicarboxylic acids Common name IUPAC name Chemical formula Structural formula Oxalic acid ethanedioic acid HOOC-COOH 
Malonic acid propanedioic acid HOOC-(CH2)-COOH 
Succinic acid butanedioic acid HOOC-(CH2)2-COOH 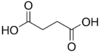
Glutaric acid pentanedioic acid HOOC-(CH2)3-COOH 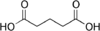
Adipic acid hexanedioic acid HOOC-(CH2)4-COOH 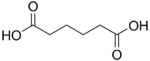
Pimelic acid heptanedioic acid HOOC-(CH2)5-COOH 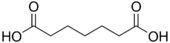
Suberic acid octanedioic acid HOOC-(CH2)6-COOH 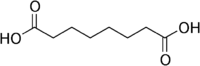
Azelaic acid nonanedioic acid HOOC-(CH2)7-COOH 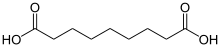
Sebacic acid decanedioic acid HOOC-(CH2)8-COOH 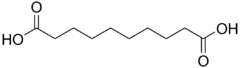
undecanedioic acid HOOC-(CH2)9-COOH dodecanedioic acid HOOC-(CH2)10-COOH Elementary aromatic dicarboxylic acids Common name IUPAC name Chemical formula Structural formula (Ortho-)Phthalic acid benzene-1,2-dicarboxylic acid
o-phthalic acidC6H4(COOH)2 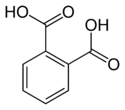
Isophthalic acid benzene-1,3-dicarboxylic acid
m-phthalic acidC6H4(COOH)2 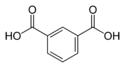
Terephthalic acid benzene-1,4-dicarboxylic acid
p-phthalic acidC6H4(COOH)2 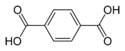
Elementary unsaturated dicarboxylic acids Type Common name IUPAC name Chemical formula Structural formula Monounsaturated: two isomeric forms: cis and trans Maleic acid (cis form) and Fumaric acid (trans form) (Z)-Butenedioic acid and (E)-Butenedioic acid HO2CCH=CHCO2H Glutaconic acid Pent-2-enedioic acid HO2CCH=CHCH2CO2H Traumatic acid Dodec-2-enedioic acid HO2C(CH2)8CH=CHCO2H Diunsaturated: three isomeric forms: trans,trans, cis,trans and cis,cis Muconic acid (2E,4E)-Hexa-2,4-dienedioic acid HO2CCH=CHCH=CHCO2H See also
More on Dibasic or Dicarboxylic Acids
Although the dicarboxylic acids do not occur in appreciable amounts as components of animal or vegetal lipids, they are in general important metabolic products of fatty acids since they originate from byoxidation. Dicarboxylic acids are suitable substrates for preparation of organic acids for the pharmaceutical and food industries. Furthermore, they are useful materials for the preparation of fragrances, polyamides, adhesives, lubricants, and polyesters.
They have the general type formula HOOC-(CH2)n-COOH In vegetal, a great variety of molecular forms of dicarboxylic acids are found:
- simple forms with a straight carbon chain or a branched chain
- complex forms with a dicarboxylic acid and an alkyl side chain like alkylitaconates
Simple forms of dicarboxylic acids
Short-chain dicarboxylic acids are of great importance in the general metabolism and up to n=3 they cannot be considered as lipids since their water solubility is important. The simplest of these intermediates is oxalic acid (n=0), the others are malonic (n=1), succinic (n=2) and glutaric (n=3) acids.
The other lipid members of the group found in natural products or from synthesis have a "n" value from 4 up to 21.
- Adipic acid (n=4) : Despite its name (in Latin adipis is fat), this acid (hexanedioic acid) is not a normal constituent of natural lipids but is a product of oxidative rancidity (lipid peroxidation). It was obtained [1] by oxidation of castor oil with nitric acid (splitting of the carbon chain close to the OH group). Synthesized in 1902 from tetramethylene bromide, it is now obtained by oxidation of cyclohexanol or cyclohexane. It has several industrial uses in the production of adhesives, plasticizers, gelatinizing agents, hydraulic fluids, lubricants, emollients, as an additive in the manufacture of some form of nylon (nylon-6,6), polyurethane foams, leather tanning, urethane and also as an acidulant in foods. Adipic acid is used after esterification with various groups such as dicapryl, di(ethylhexyl), diisobutyl, and diisodecyl.
- Pimelic acid (n=5) : this acid (heptanedioic acid), from the Greek pimelh (pimele fat), as adipic acid, was isolated from oxidized fats. It was obtained in 1884 by Ganttner F et al.[2] as a product of ricinoleic acid (hydroxylated oleic acid) from castor oil.
- Suberic acid (n=6) : it was firstly produced by nitric acid oxidation of cork (Latin suber) material and then from castor oil [3]. The oxidation of ricinoleic acid produces, by splitting at the level of the double bond and at the level of the OH group, at the same time, suberic acid (octanedioic acid) and the next homologue azelaic acid. Suberic acid was used in the manufacture of alkyd resins and in the synthesis of polyamides leading to nylon.
- Azelaic acid (n=7) : nonanedioic acid is the best known dicarboxylic acid. Its name stems from the action of nitric acid (azote, nitrogen, or azotic, nitric) oxidation of oleic or elaidic acid. It was detected among products of rancid fats [4]. Its origin explains for its presence in poorly preserved samples of linseed oil and in specimens of ointment removed from Egyptian tombs 5000 years old [5]. Azelaic acid was prepared by oxidation of oleic acid with potassium permanganate [6], but now by oxidative cleavage of oleic acid with chromic acid or by ozonolysis. Azelaic acid is used, as simple esters or branched-chain esters) in the manufacture of plasticizers (for vinyl chloride resins, rubber), lubricants and greases. Azelaic acid is now used in cosmetics (treatment of acne). It displays bacteriostatic and bactericidal properties against a variety of aerobic and anaerobic micro-organisms present on acne-bearing skin. . Azelaic acid was identified as a molecule that accumulated at elevated levels in some parts of plants and was shown to be able to enhance the resistance of plants to infections [7].
- Sebacic acid (n=8) : decanedioic acid was named by Thenard LJ (1802) from the Latin sebaceus(tallow candle) or sebum (tallow) in reference to its use in the manufacture of candles. Thenard LJ isolated this compound from distillation products of beef tallow. In 1954, it was reported that it was produced in excess of 10,000 tons annually by alkali fission of castor oil [8]. Sebacic acid and its derivatives, as azelaic acid, have a variety of industrial uses as plasticizers, lubricants, diffusion pump oils, cosmetics, candles, etc. It is also used in the synthesis of polyamide, as nylon, and of alkyd resins. An isomer, isosebacic acid, has several applications in the manufacture of vinyl resin plasticizers, extrusion plastics, adhesives, ester lubricants, polyesters, polyurethane resins and synthetic rubber.
- Dodecanedioic acid (n=10) : that acid is used in the production of nylon (nylon-6,12), polyamides, coatings, adhesives, greases, polyesters, dyestuffs, detergents, flame retardants, and fragrances. It is now produced by fermentation of long-chain alkanes with a specific strain of Candida tropicalis [9]. Its monounsaturated analogue (traumatic acid) is described below.
It was shown that all these dicarboxylic acids are formed during the drying process of paint oils and that the determination of these decomposition products may be of value in determining the age of old samples.
The higher weight dicarboxylic acids (n=10-21) are found in different plant lipids, particularly in what was named erroneously Japan wax (triglycerides containing C20, C21, C22 and C23 dicarboxylic acids besides normal fatty acids) from the sumac tree (Rhus sp.). Among them, Thapsic acid (n=14) was isolated from the dried roots of the Mediterranean "deadly carrot", Thapsia garganica (Umbelliferae), but others, as Brassylic acid (n=11), were prepared chemically from different sources. Brassylic acid can be produced chemically from erucic acid by ozonolysis but also by microorganisms (Candida sp.) from tridecane. This diacid is produced on a small commercial scale in Japan for the manufacture of fragrances. [10]
A large survey of the dicarboxylic acids present in Mediterranean nuts revealed unusual components [11]. A total of 26 minor acids (from 2 in pecan to 8% in peanut) were determined : 8 species derived from butanedioic acid, likely in relation with photosynthesis, and 18 species with a chain from 5 to 22 carbon atoms.
Higher weight acids (>C20) are found in suberin present at vegetal surfaces (outer bark, root epidermis). C16 to C26 a, w-dioic acids are considered as diagnostic for suberin. With C18:1 and C18:2, their content amount from 24 to 45% of whole suberin. They are present at low levels (< 5%) in plant cutin, except in Arabidopsis thaliana where their content can be higher than 50% [12].
The first allenic dicarboxylic acid, named glutinic acid (2,3-pentadienedioic acid) was isolated from Alnus glutinosa (Betulaceae) [13].
It was shown that hyperthermophilic microorganisms specifically contained a large variety of dicarboxylic acids [14]. This is probably the most important difference between these microorganisms and other marine bacteria. Dioic fatty acids from C16 to C22 were found in an hyperthermophilic archaeon, Pyrococcus furiosus. Short and medium chain (up to 11 carbon atoms) dioic acids have been discovered in Cyanobacteria of the genus Aphanizomenon [15].
A monounsaturated dicarboxylic acid, traumatic acid, (10E-dodeca-1,12-dicarboxylic acid), was among the first biologically active molecules isolated from plant tissues [16]. That dicarboxylic acid was shown to be a potent wound healing agent in plant that stimulates cell division near a wound site [17], it derives from 18:2 or 18:3 fatty acid hydroperoxides after conversion into oxo fatty acids. While polyunsaturated fatty acids are unusual in plant cuticles, a diunsaturated dicarboxylic acid has been reported as a component of the surface waxes or polyesters of some plant species. Thus, octadeca-c6,c9-diene-1,18-dioate, a derivative of linoleic acid, is present in Arabidopsis and Brassica napus cuticle [18].
Dicarboxylic acids were shown in 1934 to be produced by w-oxidation of fatty acids during their catabolism [19]. It was discovered that these compounds appeared in urine after administration of tricaprin and triundecylin. Although the significance of their biosynthesis remains poorly understood, it was demonstrated that w-oxidation occurs in rat liver but at a low rate, needs oxygen, NADPH and cytochrome P450. It was later shown that this reaction is more important in starving or diabetic animals where 15% of palmitic acid is subjected to w-oxidation and then tob-oxidation [20], this generates malonyl-coA which is further used in saturated fatty acid synthesis.
It was proposed recently that dicarboxylic acids are alternate lipid substrates in parenteral nutrition [21]. Basically, they are water soluble, undergo b-oxidation, do not induce ketogenesis but rather promote gluconeogenesis. They could represent an immediately available form of energy. Thus, inorganic salts of sebacic (C10) and lauric (C12) acids were firstly proposed, but now, triglycerides containing these fatty acids are under investigation [22]. Treatment of rats with derivatives of C16 dioic acid have shown that this compound markedly improved lipid metabolism [23] and inhibited the development of advanced cardiovascular disease [24].
It must be recalled that the determination of the dicarboxylic acids generated by permanganate-periodate oxidation of monoenoic fatty acids was useful to study the position of the double bond in the carbon chain [25].
Branched-chain diacids
Long-chain dicarboxylic acids containing vicinal dimethyl branching near the centre of the carbon chain have been discovered in the genus Butyrivibrio, bacteria which participate in the digestion of cellulose in the rumen [26]. These fatty acids, named diabolic acids, have a chain length depending on the fatty acid used in the culture medium. The most abundant diabolic acid inButyrivibrio had a 32-carbon chain length.
Diabolic acid (15,16-dimethyltriacontanedioic acid) These diacids were also detected in the core lipids of the genus Thermotoga of the order Thermotogales, bacteria living in solfatara springs, deep-sea marine hydrothermal systems and high-temperature marine and continental oil fields [27]. It was shown that about 10% of their lipid fraction were symmetrical C30 to C34 diabolic acids. The C30 (13,14-dimethyloctacosanedioic acid) and C32 (15,16-dimethyltriacontanedioic acid) diabolic acids have been described in Thermotoga maritima [28]. Some parent C29 to C32 diacids but with methyl groups on the carbons C-13 and C-16 have been isolated and characterized from the lipids of thermophilic anaerobic eubacterium Themanaerobacter ethanolicus[29]. The most abundant diacid was the C30 a,w-13,16-dimethyloctacosanedioic acid.
Biphytanic diacids are present in geological sediments and are considered as tracers of past anaerobic oxidation of methane [30]. Several forms without or with one or two pentacyclic rings have been detected in Cenozoic seep limestones. These lipids may be unrecognized metabolites from Archaea.
Crocetin is the core compound of crocins (crocetin glycosides) which are the main red pigments of the stigmas of saffron (Crocus sativus) and the fruits of gardenia (Gardenia jasminoides). Crocetin is a 20-carbon chain dicarboxylic acid which is a diterpenenoid and can be considered as a carotenoid. It was the first plant carotenoid to be recognized as early as 1818 while the history of saffron cultivation reaches back more than 3,000 years. The major active ingredient of saffron is the yellow pigment crocin 2 (three other derivatives with different glycosylations are known) containing a gentiobiose (disaccharide) group at each end of the molecule.
A simple and speccific HPLC-UV method has been developed to quantify the five major biologically active ingredients of saffron, namely the four crocins and crocetin [31].
Alkylitaconates
Several dicarboxylic acids having an alkyl side chain and an itaconate core have been isolated from lichens and fungi, itaconic acid (methylenesuccinic acid) being a metabolite produced by filamentous fungi.
Among these compounds, several analogues, called chaetomellic acids with different chain lengths and degrees of unsaturation have been isolated from various species of the lichen Chaetomella.
These molecules were shown to be valuable as basis for the development of anticancer drugs due to their strong farnesyltransferase inhibitory effects [32].
In 1999, a series of new fungal alkyl- and alkenyl-itaconates, ceriporic acids, were found in cultures of a selective lignin-degrading fungus (white rot fungus), Ceriporiopsis subvermispora [33].
It was determined that these ceriporic acids suppressed iron redox reactions to attenuate •OH production by the Fenton reaction in the presence of iron reductants such as hydroquinone and cysteine [34]. It was proposed that the suppression of the cellulolytic active oxygen species, •OH, by this metabolite contributes to the selective lignin-degradation with a minimum loss of cellulose. The absolute configuration of ceriporic acids, their stereoselective biosynthetic pathway and the diversity of their metabolites have been largely discussed [35].
Fatty acid carbonates
Carbonates (esters of carbonic acid, H2CO3) are well known to chemists as they represent an important class of organic compounds and among them oleochemical carbonates have interesting characteristics which make them candidates for many industrial applications.
The most common carbonates have the following structure: RO—CO—OR. R is a linear chain with 8 to 18 carbon atoms, saturated or with one double bond (dioleyl carbonate), or a branched chain (ethylhexyl, butyloctyl, or hexyldecyl).
They are miscible in organic solvents but insoluble in water. Unsaturation or branching on the alkyl chain lowers their melting point [36]. The condensation of phosgene (ClCOCl) with an alcohol appears the most commonly used procedure to synthesize oleochemical carbonates.
The polar nature of the carbonate moiety enables it to adhere strongly to metal surfaces. Thus, they are used as lubricant components which have a protective property for metal corrosion. Some C8 to C18 carbonates have been exploited in personal-care products (sunscreen, cosmetics), dioctyl carbonate being also used as emollient or solvent in UV-filter solutions. Extraction of metal ions (gold, silver, platinum) is improved by the use of the chelating properties of oleochemical carbonates when mixed with the metal-containing aqueous phase. Future developments will ensure a growing interest in these molecules.
Phenyl and benzoic alkanoic acids
Short chain w-phenylalkanoic acids have long been known to occur in natural products. Phenylacetic, 3-phenylpropanoic and 3-phenylpropenoic (cinnamic) acids were found in propolis, mammalian exocrine secretions or plant fragrances. During a systematic study of the lipids from seeds of the plant Araceae,[37] the presence of 13-phenyltridecanoic acid as a major component (5-16% of total fatty acids)was discovered. Other similar compounds but with 11 and 15 carbon chain lengths and saturated or unsaturated were shown to be also present but in lower amounts. At the same time, the even carbon chainw-phenylalkanoic acids of C10 up to C16 were discovered in halophilic bacteria [38].
w-phenylalkanoic acid (x = 1 to 17)
Later, an exhaustive study of 17 genus of the subfamily Aroideae of Araceae revealed the presence of three major acids, 11-phenylundecanoic acid, 13-phenyltridecanoic acid and 15-phenylpentadecanoic acid in seed lipids [39]. Other odd carbon number acids from C7 to C23 were detected but in trace amounts. Similarly, two series of homologous odd carbon number monounsaturated w-phenylalkanoic acids were found. Thus, it can be stated that all odd carbon chain w-phenylalkanoic acids from C1 through C23 have been found in nature. Furthermore, even carbon chain w-phenylalkanoic acids from C10 through C16 were also detected.
Substituted phenylalkenoic acids are periodically encountered in nature. As an example, rubrenoic acids were purified from Alteromonas rubra, compounds which showed bronchodilatatoric properties [40].
Methyl phenylalkenoic acids (5 carbon chain) have been described from a terrestrial Streptomycete [41]. Serpentene, a similar polyunsaturated phenylalkenoic acid, is also produced by Streptomyces and was shown to have some antibacterial properties.
Several serpentene-like compounds have also been isolated from the same bacterial source.[42]
Several bicyclic derivatives of linolenic acid were shown to be generated by alkali isomerization.[43]
Bicyclic hexahydroindenoic acid
Some others (alkyl-phenyl)-alkanoic acids) are formed when linolenic acid is warmed at 260–270°C.[44]
Several forms with 16, 18 and 20 carbon atoms were identified in archaeological pottery vessels and were presumed to have been generated during heating triunsaturated fatty acids. They were used as biomarkers to trace the ancient processing of marine animal in these vessels.[45]
Several benzoic acid derivatives have been described in leaves of various Piperaceae species. Thus, a prenylated benzoic acid acid derivative, crassinervic acid, has been isolated from Piper crassinervium.[46]
Crassinervic acid
Similar compounds were isolated from Piper aduncum (aduncumene) and Piper gaudichaudianum(gaudichaudianic acid). All these molecules showed high potential as antifungal compounds. A prenylated benzoic acid with a side chain formed of two isoprene units has also been isolated from the leaves of Piper aduncum [47]. More recently, three prenylated benzoic acid derivatives with four isoprene units have been extracted from the leaves of Piper heterophyllum and Piper aduncum [48]. These compounds displayed moderate antiplasmodial (against Plasmodium falciparum) and trypanocidal (against Trypanosoma cruzi) activities.
Fatty acyl-CoA esters
These fatty acid derivatives may be considered as complex lipids since they are formed of one fatty acid, a 3'-phospho-AMP linked to phosphorylated pantothenic acid (Vitamin F) and cysteamine. However, to simplify the nomenclature and taking into account their metabolism, we classify them within the big group of the fatty acids and their simple derivatives rather than within the complex and phosphorylated lipids. Long-chain acyl-CoA esters are substrates for a number of important enzymatic reactions and play a central role in the regulation of metabolism as allosteric regulators of several enzymes. To participate in specific metabolic processes, fatty acids must first be activated by being joined in thioester linkage (R-CO-SCoA) to the -SH group of coenzyme A. The thioester bond is a high energy bond.
R = fatty carbon chain
The activation reaction normally occurs in the endoplasmic reticulum or the outer mitochondrial membrane. This is an ATP-requiring reaction (fatty acyl-CoA synthase), yielding AMP and pyrophosphate (PPi). Different enzymes are specific for fatty acids of different chain length. Then, the acyl CoA esters are transported in mitochondria. They are converted to fatty acyl carnitine by carnitine acyl transferase I, an enzyme of the inner leaflet of the outer mitochondrial membrane. Fatty acyl carnitine is then transported by an antiport in exchange for free carnitine to the inner surface of the inner mitochondrial membrane. There carnitine acyl transferase II reverses the process, producing fatty acyl-CoA and carnitine. This shuttle mechanism is required only for longer chain fatty acids. Once inside the mitochondrial matrix, the fatty acyl-CoA derivatives are degraded by a series of reactions that release acetyl-CoA and leads to the production of NADH and FADH2. There are four steps in fatty acid oxidation pathway; oxidation, hydration, oxidation, and thiolysis. It requires 7 rounds of this pathway to degrade palmitate (a C16 fatty acid).
Divinyl ether fatty acids
Fatty acid hydroperoxides generated by plant lipoxygenases from linoleic and linolenic acids are known to serve as substrates for a divinyl ether synthase which produces divinyl ether fatty acids. Up to date divinyl ethers were detected only within the plant kingdom. The discovery of that class of compounds dates back to 1972, when the structures of two ether C18 fatty acids generated by homogenates of the potato tuber wee described [49]. These compounds, named colneleic acid (from linoleic acid) and colnelenicacid (from linolenic acid), could be also produced in potato leaves and tomato roots by rearrangement of 9-hydroperoxides.
Isomers of colneleic and colnelenic acids were isolated from homogenates of leaves of Clematis vitalba (Ranunculaceae) [50].
Similarly, 13-lipoxygenase-generated hydroperoxides serve as precursor of other divinyl ether fatty acids which are produced in bulbs of garlic [51] or Ranunculus leaves [52]. These compounds were named etheroleic and etherolenic acids.
The physiological significance of divinyl ethers is still not fully studied. As infection of potato leaves leads to increased levels of divinyl ether synthase, it was suggested that this pathway could be of importance in the defense of plants against attacking pathogens [53]. Similar structures have been discovered in the brown alga Laminaria sinclairii, with 18 or 20 carbons and 4, 5 or 6 double bonds [54], and in the red alga Polyneura latissima, with 20 carbons and 5 double bonds [55].
References
- ^ Dieterle W et al. Berichte der Deutschen Chemischen Gesellschaft 1884, 17, 2221
- ^ Berichte der Deutschen Chemischen Gesellschaft. 1884, 17, 2212
- ^ Tilley TG, Ann 1841, 39, 160
- ^ Nicolet BH et al., Journal Industrial Engineering Chemistry 1916, 8, 416 and Nunn L et al., Biochemistry Journal 1938, 32, 1974
- ^ Banks A et al., Analyst 1933, 58, 265
- ^ Ganttner F et al., Berichte der Deutschen Chemischen Gesellschaft 1881, 14, 1545
- ^ Jung HW et al., Science (journal) 2009, 324, 89)
- ^ Kadesch RG, Journal of the American Oil Chemists' Society (JAOCS) 1954, 31, 568
- ^ Kroha K, Inform 2004, 15, 568
- ^ A review on the applications and the industrial biotechnology of these molecules has been released by Kroha K, Inform 2004, 15, 568.
- ^ Dembitsky VM et al., Food Chemistry 2002, 76, 469
- ^ Pollard Met al., Trends in Plant Science 2008, 13, 236
- ^ Hans EA, Berichte der Deutschen Chemischen Gesellschaft 1908, 40, 4760
- ^ Carballeira NM et al., Journal Bacteriology 1997, 179, 2766
- ^ Dembitsky VM et al., Biochemistry (Moscow) 2001, 66, 72
- ^ English J et al., Science (journal) 1939, 90, 329
- ^ Farmer EE, Plant Molecular Biology 1994, 26, 1423
- ^ Bonaventure G et al., Plant Journal 2004, 40, 920
- ^ Verkade PE et al., Biochemical Journal 1934, 28, 31
- ^ Wada F et al. Biochimica et Biophysica Acta 1977, 487, 261
- ^ Greco AV et al., Clinical Nutrition 1995, 14, 143
- ^ Capristo E et al. Clinica Chimica Acta 1999, 289, 11
- ^ Russell JC et al., Arteriosclorosis, Thrombosis and Vascular Biology 1991, 11, 602
- ^ Russell JC et al., Arterioclorosis Thrombosis and Vascular Biology 1995, 15, 918
- ^ Longmuir KJ et al., Analytical Biochemistry (journal) 1987, 167, 213
- ^ Klein RA et al., Biochem J 1979, 183, 691
- ^ Huber R et al., Arch Microbiol 1986, 144, 324
- ^ Caballeira NM et al., Journal Bacteriology 1997, 179, 2766
- ^ Jung S et al., Journal Lipid Research 1994, 35, 1057
- ^ Birgel D et al., Org Geochemistry 2008, 39, 152
- ^ Li N et al., Journal Chromatography A 1999, 849, 349
- ^ Singh SB et al., Bioorg Med Chem 2000, 8, 571
- ^ Enoki M et al., Chem Lett 2000, 54-55, Amirta R et al., Chem Phys Lipids 2003, 126, 121
- ^ Enoki M et al., Chem Phys Lipids 2002, 120, 9
- ^ Nishimura H et al., Chem Phys Lipids 2009, 159, 77
- ^ Kenar JA, Inform 2004, 15, 580
- ^ Schmid PC et al. Phytochemistry 1997, 45, 1173
- ^ Caballeira NM et al., Lipids 1997, 32, 1271
- ^ Meija J et al., Phytochemistry 2004, 65, 2229
- ^ Holland GS et al., Chem Ind 1984, 850
- ^ Mukku VJ et al., Z Naturforsch 2002, 57b, 335
- ^ Wenzel SC et al., J Nat Prod 2004, 67, 1631
- ^ Matikainen J et al., Tetrahedron Lett 2003, 59, 567
- ^ Hase A et al., JAOCS 1978, 55, 407
- ^ Hansel FA et al., Tetrahedron Lett 2004, 45, 2999
- ^ Lago JH et al., J Nat Prod 2004, 67, 1783
- ^ Baldoqui DC et al., Phytochemistry 1999, 51, 899
- ^ Flores N et al., Phytochemistry 2009, 70, 621
- ^ Galliard T et al.,Biochem J 1972, 129, 743
- ^ Hamberg M, Lipids 2004, 39, 565
- ^ Grechkin AN et al., FEBS Lett 1995, 371, 159
- ^ Hamberg M, Lipids 1998, 33, 1061
- ^ Göbel C et al., Biochim Biophys Acta 2002, 1584, 55
- ^ Proteau PJ et al., Lipids 1993, 28, 783
- ^ Jiang ZD et al., Lipids 1997, 32, 231
Categories:- Dicarboxylic acids
Wikimedia Foundation. 2010.
