- Lichen
-
 Lichen-covered tree: Grey, leafy Parmotrema perlatum on upper half of trunk; yellowy-green Flavoparmelia caperata on middle and lower half and running up the extreme right side; and the fruticose Ramalina farinacea. Tresco, Isles of Scilly, UK
Lichen-covered tree: Grey, leafy Parmotrema perlatum on upper half of trunk; yellowy-green Flavoparmelia caperata on middle and lower half and running up the extreme right side; and the fruticose Ramalina farinacea. Tresco, Isles of Scilly, UK
Lichens (
 /ˈlaɪkən/,[1] sometimes /ˈlɪtʃən/)[2] are composite organisms consisting of a symbiotic organism composed of a fungus (the mycobiont) with a photosynthetic partner (the photobiont or phycobiont), usually either a green alga (commonly Trebouxia) or cyanobacterium (commonly Nostoc).[3] The morphology, physiology and biochemistry of lichens are very different from those of the isolated fungus and alga in culture. Lichens occur in some of the most extreme environments on Earth—arctic tundra, hot deserts, rocky coasts, and toxic slag heaps. However, they are also abundant as epiphytes on leaves and branches in rain forests and temperate woodland, on bare rock, including walls and gravestones, and on exposed soil surfaces (e.g., Collema) in otherwise mesic habitats. Lichens are widespread and may be long-lived;[4] however, many are also vulnerable to environmental disturbance, and may be useful to scientists in assessing the effects of air pollution,[5][6][7] ozone depletion, and metal contamination. Lichens have also been used in making dyes and perfumes, as well as in traditional medicines.
/ˈlaɪkən/,[1] sometimes /ˈlɪtʃən/)[2] are composite organisms consisting of a symbiotic organism composed of a fungus (the mycobiont) with a photosynthetic partner (the photobiont or phycobiont), usually either a green alga (commonly Trebouxia) or cyanobacterium (commonly Nostoc).[3] The morphology, physiology and biochemistry of lichens are very different from those of the isolated fungus and alga in culture. Lichens occur in some of the most extreme environments on Earth—arctic tundra, hot deserts, rocky coasts, and toxic slag heaps. However, they are also abundant as epiphytes on leaves and branches in rain forests and temperate woodland, on bare rock, including walls and gravestones, and on exposed soil surfaces (e.g., Collema) in otherwise mesic habitats. Lichens are widespread and may be long-lived;[4] however, many are also vulnerable to environmental disturbance, and may be useful to scientists in assessing the effects of air pollution,[5][6][7] ozone depletion, and metal contamination. Lichens have also been used in making dyes and perfumes, as well as in traditional medicines.Contents
Overview
The body (thallus) of most lichens is quite different from those of either the fungus or alga growing separately, and may strikingly resemble simple plants in form and growth. The fungus surrounds the algal cells, often enclosing them within complex fungal tissues unique to lichen associations. In many species the fungus penetrates the algal cell wall, forming penetration pegs or haustoria similar to those produced by pathogenic fungi.[3][8] Lichens are poikilohydric, capable of surviving extremely low levels of water content.[9] However, the re-configuration of membranes following a period of dehydration requires several minutes at least. During this period a “soup” of metabolites from both the mycobiont and phycobiont leaks into the extracellar spaces. This is readily available to both bionts to take up essential metabolic products ensuring a near perfect level of mutualism.[citation needed] Other epiphytic organisms may also benefit from this nutrient rich leachate.[citation needed] This phenomenon also points to a possible explanation of lichen evolution from its original phycobiont and mycobiont components with its subsequent migration from an aquatic environment to dry land.[citation needed]
The algal or cyanobacterial cells are photosynthetic, and as in plants they reduce atmospheric carbon dioxide into organic carbon sugars to feed both symbionts. Both partners gain water and mineral nutrients mainly from the atmosphere, through rain and dust. The fungal partner protects the alga by retaining water, serving as a larger capture area for mineral nutrients and, in some cases, provides minerals obtained from the substrate. If a cyanobacterium is present, as a primary partner or another symbiont in addition to green alga as in certain tripartite lichens, they can fix atmospheric nitrogen, complementing the activities of the green alga.
Algal and fungal components of some lichens have been cultured separately under laboratory conditions[citation needed], but in the natural environment of a lichen, neither can grow and reproduce without a symbiotic partner.[citation needed] Indeed, although strains of cyanobacteria found in various cyanolichens are often closely related to one another, they differ from the most closely related free-living strains.[10] The lichen association is a close symbiosis: It extends the ecological range of both partners and is obligatory for their growth and reproduction in natural environments.[citation needed] Propagules (diaspores) typically contain cells from both partners, although the fungal components of so-called "fringe species" rely instead on algal cells dispersed by the “core species.”[citation needed]
Lichen associations may be considered as examples of mutualism, commensalism or even parasitism, depending on the species. Cyanobacteria in laboratory settings can grow faster when they are alone rather than when they are part of a lichen. The same, however, might be said of isolated skin cells growing in laboratory culture, which grow more quickly than similar cells that are integrated into a functional tissue. However, from the work of Coxson mutualism would appear to best summarise our current knowledge.
History
Although lichens had been recognized as organisms for quite some time, it was not until 1867, when Swiss botanist Simon Schwendener proposed his dual theory of lichens, that the true nature of the lichen association began to emerge.[11] Schwendener's hypothesis, which at the time lacked experimental evidence, arose from his extensive analysis of the anatomy and development in lichens, algae, and fungi using a light microscope. Many of the leading lichenologists at the time, such as James Crombie and Nylander, rejected Schwendener's hypothesis because the common consensus was that all living organisms were autonomous.[11] Other prominent biologists, such as Heinrich Anton de Bary, Albert Bernhard Frank, and Hermann Hellriegel were not so quick to reject Schwendener's ideas and the concept soon spread into other areas of study, such as microbial, plant, animal and human pathogens.[11] When the complex relationships between pathogenic microorganisms and their hosts were finally identified—refuting the idea of holistic organisms—Schwendener's hypothesis began to gain popularity. Further experimental proof of the dual nature of lichens was obtained when Eugen Thomas published his results in 1939 on the first successful re-synthesis experiment.[11]
Symbionts
Living as a symbiont in a lichen appears to be a very successful way for a fungus to derive essential nutrients, as about 20% of all fungal species have acquired this mode of life. The largest number of lichenized fungi occur in the Ascomycota, with about 40% of species forming such an association.[12] Some of these lichenized fungi occur in orders with nonlichenized fungi that live as saprotrophs or plant parasites (for example, the Leotiales, Dothideales, and Pezizales). Other lichen fungi occur in only five orders in which all members are engaged in this habit (Orders Graphidales, Gyalectales, Peltigerales, Pertusariales, and Teleoschistales). Lichenized and nonlichenized fungi can even be found in the same genus or species. Overall, about 98% of lichens have an ascomycetous mycobiont . Next to the Ascomycota, the largest number of lichenized fungi occur in the unassigned fungi imperfecti. Comparatively few Basidiomycetes are lichenized, but these include agarics, such as species of Lichenomphalia, clavarioid fungi, such as species of Multiclavula, and corticioid fungi, such as species of Dictyonema.
The autotrophic symbionts occurring in lichens are simple, photosynthetic organisms commonly and traditionally known as algae. These symbionts include both prokaryotic and eukaryotic organisms. Approximately 100 species of photosynthetic partners from 40 genera and five distinct classes (prokaryotic: Cyanphyceae; eukaryotic: Tribophyceae, Phaephyceae, Chlorophyceae, and Pleurostrophyceae) have been found to associate with the lichen-forming fungi.[13] The prokaryotes belong to the Cyanobacteria, whose representatives are often called bluegreen algae. The bluegreen algae occur as symbionts in about 8% of the known lichens. The most commonly occurring genus is Nostoc.[14] The majority of the lichens contain eukaryotic autotrophs belonging to the Chlorophyta (green algae) or to the Xanthophyta (yellow-green algae). About 90% of all known lichens have a green algae as a symbiont, and among these, Trebouxia is the most common genus, occurring in about 40% of all lichens. The second most commonly represented green algae genus is Trentepohlia. Overall, about 100 species are known to occur as autotrophs in lichens. All the algae are probably able to exist independently in nature as well as in the lichen.[14]
A particular fungus species and algal species are not necessarily always associated together in a lichen. One fungus, for example, can form lichens with a variety of different algae. The thalli produced by a given fungal symbiont with its differing partners will be similar, and the secondary metabolites identical, indicating that the fungus has the dominant role in determining the morphology of the lichen. Further, the same algal species can occur in association with different fungal partners. Lichens are known in which there is one fungus associated with two or even three algal species. Rarely, the reverse can occur, and two or more fungal species can interact to form the same lichen.[14]
Both the lichen and the fungus partner bear the same scientific name, and the lichens are being integrated into the classification schemes for fungi. The alga bears its own scientific name, which bears no relationship to that of the lichen or fungi.[12]
Morphology and structure
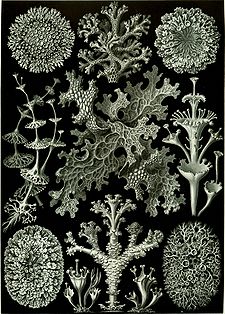
Some lichens have the aspect of leaves (foliose lichens); others cover the substrate like a crust (crustose lichens) (illustration, right), others such as the genus Ramalina adopt shrubby forms (fruticose lichens), and there are gelatinous lichens such as the genus Collema.[15]
Although the form of a lichen is determined by the genetic material of the fungal partner, association with a photobiont is required for the development of that form. When grown in the laboratory in the absence of its photobiont, a lichen fungus develops as an undifferentiated mass of hyphae. If combined with its photobiont under appropriate conditions, its characteristic form emerges, in the process called morphogenesis (Brodo, Sharnoff & Sharnoff, 2001). In a few remarkable cases, a single lichen fungus can develop into two very different lichen forms when associating with either a green algal or a cyanobacterial symbiont. Quite naturally, these alternative forms were at first considered to be different species, until they were first found growing in a conjoined manner.
There is evidence to suggest that the lichen symbiosis is parasitic or commensalistic, rather than mutualistic (Ahmadjian 1993). However, this now needs to be re-examined in light of Coxon's work. The photosynthetic partner can exist in nature independently of the fungal partner, but not vice versa. Furthermore, photobiont cells are routinely destroyed in the course of nutrient exchange. The association is able to continue because reproduction of the photobiont cells matches the rate at which they are destroyed. (ibid.)
Under magnification, a section through a typical foliose lichen thallus reveals four layers of interlaced fungal filaments. The uppermost layer is formed by densely agglutinated fungal hyphae building a protective outer layer called the cortex, which can reach several hundred μm in thickness.[16] This cortex may be further topped by an epicortex 0.6-1μm thick in some Parmeliaceae, which may be with or without pores, and is secreted by cells—it is not itself cellular.[16] In lichens that include both green algal and cyanobacterial symbionts, the cyanobacteria may be held on the upper or lower surface in small pustules called cephalodia. Beneath the upper cortex is an algal layer composed of algal cells embedded in rather densely interwoven fungal hyphae. Each cell or group of cells of the photobiont is usually individually wrapped by hyphae, and in some cases penetrated by an haustorium. Beneath this algal layer is a third layer of loosely interwoven fungal hyphae without algal cells. This layer is called the medulla. Beneath the medulla, the bottom surface resembles the upper surface and is called the lower cortex, again consisting of densely packed fungal hyphae. The lower cortex often bears rootlike fungal structures known as rhizines, which serve to attach the thallus to the substrate on which it grows. Lichens also sometimes contain structures made from fungal metabolites, for example crustose lichens sometimes have a polysaccharide layer in the cortex. Although each lichen thallus generally appears homogeneous, some evidence seems to suggest that the fungal component may consist of more than one genetic individual of that species. This seems to also be true of the photobiont species involved.
Growth form
Lichens are informally classified by growth form into:
- crustose (paint-like, flat), e.g., Caloplaca flavescens
- filamentous (hair-like), e.g., Ephebe lanata
- foliose (leafy), e.g., Hypogymnia physodes
- fruticose (branched), e.g., Cladonia evansii, C. subtenuis, and Usnea australis
- leprose (powdery), e.g., Lepraria incana
- squamulose (consisting of small scale-like structures, lacking a lower cortex), e.g., Normandina pulchella
- gelatinous lichens, in which the cyanobacteria produce a polysaccharide that absorbs and retains water.
Reproduction and dispersal
Many lichens reproduce asexually, either by vegetative reproduction or through the dispersal of diaspores containing algal and fungal cells. Soredia (singular soredium) are small groups of algal cells surrounded by fungal filaments that form in structures called soralia, from which the soredia can be dispersed by wind. Another form of diaspore are isidia, elongated outgrowths from the thallus that break off for mechanical dispersal. Fruticose lichens in particular can easily fragment. Because of the relative lack of differentiation in the thallus, the line between diaspore formation and vegetative reproduction is often blurred. Many lichens break up into fragments when they dry, dispersing themselves by wind action, to resume growth when moisture returns.
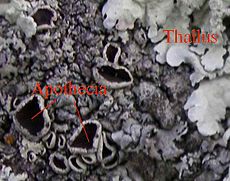
Many lichen fungi appear to reproduce sexually in a manner typical of fungi, producing spores that are presumably the result of sexual fusion and meiosis. Following dispersal, such fungal spores must meet with a compatible algal partner before a functional lichen can form. This may be a common form of reproduction in basidiolichens, which form fruitbodies resembling their nonlichenized relatives. Among the ascolichens, spores are produced in spore-producing bodies, the three most common spore body types are the apothecia, perithecia and the pycnidia.[17]
For reproduction, lichen possess isidia, soredia, and undergo simple fragmentation. These structures are also composed of a fungal hyphae wrapped around cyanobacteria. (Eichorn, Evert, and Raven, 2005) While the reproductive structures are all composed of the same components (Mycobiont and Photobiont) they are each unique in other ways. Isidia are small outgrowths on the exterior of the lichen. Soredia are powdery propagules that are released from the top of the thallus.[18] In order to establish the lichen, the soredia propagules must contain both the photobiont and the mycobiont.[19]
Growth and longevity
Lichenometry
Lichenometry is a technique used to determine the age of exposed rock surfaces based on the size of lichen thalli. Introduced by Beschel in the 1950s,[20] the technique has found many applications.
Ecology
Lichens must compete with plants for access to sunlight, but because of their small size and slow growth, they thrive in places where higher plants have difficulty growing. Lichens are often the first to settle in places lacking soil, constituting the sole vegetation in some extreme environments such as those found at high mountain elevations and at high latitudes.[citation needed] Some survive in the tough conditions of deserts, and others on frozen soil of the Arctic regions.[21]
A major ecophysiological advantage of lichens is that they are poikilohydric (poikilo- variable, hydric- relating to water), meaning that though they have little control over the status of their hydration, they can tolerate irregular and extended periods of severe desiccation. Like some mosses, liverworts, ferns, and a few "resurrection plants", upon desiccation, lichens enter a metabolic suspension or stasis (known as cryptobiosis) in which the cells of the lichen symbionts are dehydrated to a degree that halts most biochemical activity. In this cryptobiotic state, lichens can survive wider extremes of temperature, radiation and drought in the harsh environments they often inhabit.
Lichens do not have roots and do not need to tap continuous reservoirs of water like most higher plants, thus they can grow in locations impossible for most plants, such as bare rock, sterile soil or sand, and various artificial structures such as walls, roofs and monuments. Many lichens also grow as epiphytes (epi- on the surface, phyte- plant) on plants, particularly on the trunks and branches of trees. When growing on plants, lichens are not parasites; they do not consume any part of the plant nor poison it. Some ground-dwelling lichens, such as members of the subgenus Cladina (reindeer lichens), however, produce chemicals which leach into the soil and inhibit the germination of plant seeds and growth of young plants. Stability (that is, longevity) of their substrate is a major factor of lichen habitats. Most lichens grow on stable rock surfaces or the bark of old trees, but many others grow on soil and sand. In these latter cases, lichens are often an important part of soil stabilization; indeed, in some desert ecosystems, vascular (higher) plant seeds cannot become established except in places where lichen crusts stabilize the sand and help retain water.
The European Space Agency has discovered that lichens can survive unprotected in space. In an experiment led by Leopoldo Sancho from the Complutense University of Madrid, two species of lichen—Rhizocarpon geographicum and Xanthoria elegans—were sealed in a capsule and launched on a Russian Soyuz rocket on 31 May 2005. Once in orbit the capsules were opened and the lichens were directly exposed to the vacuum of space with its widely fluctuating temperatures and cosmic radiation. After 15 days the lichens were brought back to earth and were found to be in full health with no discernible damage from their time in orbit.[22][23]
When growing on mineral surfaces, some lichens slowly decompose their substrate by chemically degrading and physically disrupting the minerals, contributing to the process of weathering by which rocks are gradually turned into soil. While this contribution to weathering is usually benign, it can cause problems for artificial stone structures. For example, there is an ongoing lichen growth problem on Mount Rushmore National Memorial that requires the employment of mountain-climbing conservators to clean the monument.
Lichens may be eaten by some animals, such as reindeer, living in arctic regions. The larvae of a number of Lepidoptera species feed exclusively on lichens. These include Common Footman and Marbled Beauty. However, lichens are very low in protein and high in carbohydrates, making them unsuitable for some animals. Lichens are also used by the Northern Flying Squirrel for nesting, food, and a water source during winter.
Air pollution
Lichens are exposed to air pollutants at all times and, without any deciduous parts, they are unable to avoid the accumulation of pollutants. Also, by lacking stomata and a cuticle, aerosols and gases may be absorbed over the entire thallus surface from which they may readily diffuse to the photobiont layer.[24] Because lichens do not possess roots, their primary source of most elements is the air, and therefore elemental levels in lichens often reflect the accumulated composition of ambient air. The processes by which atmospheric deposition occurs include fog and dew, gaseous absorption, and dry deposition.[25] Consequently, many environmental studies with lichens emphasize their feasibility as effective biomonitors of atmospheric quality.[24][26]
Not all lichens are equally sensitive to air pollutants, so different lichen species show different levels of sensitivity to specific atmospheric pollutants.[27] The sensitivity of a lichen to air pollution is directly related to the energy needs of the mycobiont, so that the stronger the dependency of the mycobiont on the photobiont, the more sensitive the lichen is to air pollution.[28] Upon exposure to air pollution, the photobiont may use metabolic energy for repair of cellular structures that would otherwise be used for maintenance of photosynthetic activity, therefore leaving less metabolic energy available for the mycobiont. The alteration of the balance between the photobiont and mycobiont can lead to the breakdown of the symbiotic association. Therefore, lichen decline may result not only from the accumulation of toxic substances, but also from altered nutrient supplies that favor one symbiont over the other.[24]
Evolution and paleontology
The evolution of lichens and the phylum Ascomycota is complex and not well understood, but because there are fifteen different classes of Ascomycetes, scientists generally believe that different lichens have evolved independently from one another through analogous evolution. Lichenized fungi have continued to evolve, developing differently than those that do not form lichens.[citation needed]
Lichenization is an ancient nutritional strategy for fungi. The extreme habitats that lichens inhabit are not ordinarily conducive to producing fossils.[29] The oldest fossil lichens in which both symbiotic partners have been recovered date to the Early Devonian Rhynie chert, about 400 million years old.[30] The slightly older fossil Spongiophyton has also been interpreted as a lichen on morphological[31] and isotopic[32] grounds, although the isotopic basis is decidedly shaky.[33] It has been suggested—although not yet proven—that the even older fossil Nematothallus was a lichen.[34]
It has also been claimed that Ediacaran fossils were lichens;[35] although this claim initially met with scepticism[citation needed] for all Ediacaran fossils, additional evidence has been marshalled for a lichen interpretation of Dickinsonia.[34] Lichen-like fossils consist of coccoid cells and thin filaments, preserved in marine phosphorite of the Doushantuo Formation in southern China. These fossils are thought to be 551 to 635 million years old (belonging to the Neoproterozoic era).[36] Discovery of these fossils suggest that fungi developed symbiotic partnerships with photoautotrophs long before the evolution of vascular plants. Winfrenatia, an early zygomycetous lichen symbiosis that may have involved controlled parasitism, is an impression found in Scotland, belonging to the early Devonian times.[37] There are also several examples of fossilized lichens embedded in amber. The fossilized Anzia is found in pieces of amber in northern Europe and dates back approximately 40 million years.[38] Fossilized Lobaria comes from Trinity County in northern California, USA and dates back to the early to middle Miocene.[39]
In 1995, Gargas and colleagues proposed that there were at least five independent origins of lichenization; three in the basidiomycetes and at least two in the Ascomycetes.[40] However, Lutzoni et al. (2000) indicate that lichenization probably evolved earlier and was followed by multiple independent losses. Some non-lichen-forming fungi may have secondarily lost the ability to form a lichen association. As a result, lichenization has been viewed as a highly successful nutritional strategy.[41][42]
Lichens were a component of the early terrestrial ecosystems, and the estimated age of the oldest terrestrial lichen fossil is 400 Ma.[43][44] Recent (2009) studies suggest that the ancestral ecological state of the Ascomycota was saprobism, and that independent lichenization events have occurred multiple times.[45]
Taxonomy and classification
Lichens are named based on the fungal component, which plays the primary role in determining the lichen's form. The fungus typically comprises the majority of a lichen's bulk, though in filamentous and gelatinous lichens this is not always the case. The lichen fungus is typically a member of the Ascomycota—rarely a member of the Basidiomycota, and then termed basidiolichens to differentiate them from the more common ascolichens. Formerly, some lichen taxonomists placed lichens in their own division, the Mycophycophyta, but this practice is no longer accepted because the components belong to separate lineages. Neither the ascolichens nor the basidiolichens form monophyletic lineages in their respective fungal phyla, but they do form several major solely or primarily lichen-forming groups within each phylum.[46] Even more unusual than basidiolichens is the fungus Geosiphon pyriforme, a member of the Glomeromycota that is unique in that it encloses a cyanobacterial symbiont inside its cells. Geosiphon is not usually considered to be a lichen, and its peculiar symbiosis was not recognized for many years. The genus is more closely allied to endomycorrhizal genera.
The following table lists the orders and families of fungi that include lichen-forming species.
Taxonomy of the Lichen families Show all lichen generaAscomycota ArthoniomycetesArthonialesCapnodiaceaeDacampiaceae • XanthopyreniaceaeEpigloeaceae • Arthopyreniaceae • Didymosphaeriaceae • Lichenotheliaceae • Microthyriaceae • Mycosphaerellaceae • Naetrocymbaceae • Parmulariaceae • Pseudoperisporiaceae • Pyrenotrichaceae • ProtothelenellaceaeChaetothyriomycetidaeHerpotrichiellaceaePyrenulalesVerrucarialesAdelococcaceae • VerrucariaceaeStrigulaceaeMycocaliciaceae • SphinctrinaceaeAcarosporomycetidaeAcarosporalesAcarosporaceaeLecanoromycetidaeLecanoralesAnziaceae • Arthrorhaphidaceae • Biatorellaceae • Caliciaceae • Candelariaceae • Cetradoniaceae • Cladoniaceae • Crocyniaceae • Dactylosporaceae • Gypsoplacaceae • Haematommataceae • Lecanoraceae • Lecideaceae • Loxosporaceae • Megalariaceae • Megalosporaceae • Mycoblastaceae • Ophioparmaceae • Parmeliaceae • Physciaceae • Pilocarpaceae • Porpidiaceae • Psoraceae • Ramalinaceae • Rhizocarpaceae • Stereocaulaceae • SphaerophoraceaePeltigeralesCoccocarpiaceae • Collemataceae • Pannariaceae • Lobariaceae • Nephromataceae • Peltigeraceae • PlacynthiaceaeRhizocarpalesCatillariaceaeTeloschistalesAgyrialesAgyriaceae • Anamylopsoraceae • SchaereriaceaeCoenogoniaceae • GyalectaceaeGomphillaceae • Graphidaceae • Odontotremataceae • Solorinellaceae • Stictidaceae • ThelotremataceaePertusarialesTrichothelialesPorinaceaeArctomiaceae • HymeneliaceaeHelotiaceae • HyaloscyphaceaeLichinomycetesLichinalesGloeoheppiaceae • Heppiaceae • Lichinaceae • PeltulaceaeNitschkiaceaeHyponectriaceaeObryzaceaeLahmialesLahmiaceaeAspidotheliaceae • Mastodiaceae • Thelenellaceae • Baeomycetaceae • Coccotremataceae • ThelocarpaceaeBasidiomycota AthelialesAtheliaceae • LepidostromataceaeTremellomycetidaeTremellalesSyzygosporaceae • TremellaceaeAtractiellalesChionosphaeraceaePlatygloeaceaeReferences - Anderson, Heidi L. and Stefan Ekman. 2005. Disintegration of the Micareaceae (lichenized Ascomycota): a molecular phylogeny based on mitochondrial rDNA sequences. Mycological Research 109(1): 21–30.
- CABI Bioscience Databases. Available online at http://www.indexfungorum.org/.
- Damien Ertz, James D. Lawrey, Masoumeh Sikaroodi, Patrick M. Gillevet, Eberhard Fischer, Dorothee Killmann, and Emmanuël Sérusiaux. 2008. A new lineage of lichenized basidiomycetes inferred from a two-gene phylogeny: The Lepidostromataceae with three species from the tropics. American Journal of Botany 95(12): 1548–1556.
- Ekman, Stefan, Heidi L. Andersen, and Mats Wedin. 2008. The limitations of ancestral state reconstruction and the evolution of the ascus in the Lecanorales (lichenized Ascomycota). Systematic Biology 57(1): 141–156.
- Ekman, Stefan. 2001. Molecular phylogeny of the Bacidiaceae (Lecanorales, lichenized Ascomycota). Mycological Research 105(7): 783-797.
- Grube, Martin and Katarina Winka. 2002. Progress in understanding the evolution and classification of lichenized ascomycetes. Mycologist 16(2): 67-76.
- Liu , Yajuan J. and Benjamin D. Hall. 2004. Body plan evolution of ascomycetes, as inferred from an RNA polymerase II phylogeny. Proceedings of the National Academy of Sciences 101(13): 4507-4512.
- Schmitt I, Yamamoto Y, Lumbsch HT. 2006. Phylogeny of Pertusariales (Ascomycotina): Resurrection of Ochrolechiaceae and new circumscription of Megasporaceae. Journal of the Hattori Botanical Laboratory 100: 753-764.
- Staiger, Bettina, Klaus Kalb, and Martin Grube. 2006. Phylogeny and phenotypic variation in the lichen family Graphidaceae (Ostropomycetidae, Ascomycota). Mycological Research 110: 765-772.
Economic uses
Food
Lichens are eaten by many different cultures across the world. Although some lichens are only eaten in times of famine, others are a staple food or even a delicacy. Two obstacles are often encountered when eating lichens: lichen polysaccharides are generally indigestible to humans, and lichens usually contain mildly toxic secondary compounds that should be removed before eating. Very few lichens are poisonous, but those high in vulpinic acid or usnic acid are toxic.[47] Most poisonous lichens are yellow.
In the past Iceland moss (Cetraria islandica) was an important human food in northern Europe, and was cooked as a bread, porridge, pudding, soup, or salad. Wila (Bryoria fremontii) was an important food in parts of North America, where it was usually pitcooked. Northern peoples in North America and Siberia traditionally eat the partially digested reindeer lichen (Cladina spp.) after they remove it from the rumen of caribou or reindeer that have been killed. Rock tripe (Umbilicaria spp. and Lasalia spp.) is a lichen that has frequently been used as an emergency food in North America, and one species, Umbilicaria esculenta, is used in a variety of traditional Korean and Japanese foods.
Other Uses
Many lichens produce secondary compounds, including pigments that reduce harmful amounts of sunlight and powerful toxins that reduce herbivory or kill bacteria. These compounds are very useful for lichen identification, and have had economic importance as dyes such as cudbear or primitive antibiotics.
There are reports dating almost 2000 years of lichens being used to extract purple and red colors.[48] Of great historical and commercial significance are lichens belonging to the family Roccellaceae, commonly called orchella weed or orchil. Orcein and other lichen dyes have largely been replaced by synthetic versions. The pH indicator litmus is a dye extracted from the lichen genus Rocella tinctoria by boiling
Extracts from many Usnea species were used to treat wounds in Russia in the mid-twentieth century.[citation needed]
The substance olivetol is found to be naturally present in certain species of lichens. This is a property it shares with the cannabis plant, which internally produces the related substance olivetolic acid (before using it to biosynthesise tetrahydrocannabinol (THC)).[49]
Lichen is used in model railroading[50] and other modeling hobbies as a material for making trees and shrubs.
An article posted on PLoS one indicates that certain species of Lichen degrade prions.[51][52]
Gallery
-
Map lichen (Rhizocarpon geographicum) on rock
-
Microscopic view of lichen growing on a piece of concrete dust.[53]
See also
References
- ^ "Lichen". Oxford English Dictionary. Oxford University Press. 2nd ed. 1989.
- ^ Cambridge Advanced Learner's Dictionary Second Edition, page 731. Cambridge University Press, 2005
- ^ a b F.S. Dobson (2000) Lichens, an illustrated guide to the British and Irish species. Richmond Publishing Co. Ltd., Slough, UK
- ^ Morris J, Purvis W. (2007). Lichens (Life). London: The Natural History Museum. p. 19. ISBN 0-565-09153-0.
- ^ Ferry, B.W., Baddeley, M.S. & Hawkworth, D. L. (Editors) (1973) Air Pollution and Lichens. Athlone Press, London.
- ^ Rose C.I., Hawksworth D.L. (1981). "Lichen recolonization in London's cleaner air". Nature 289 (5795): 289–292. Bibcode 1981Natur.289..289R. doi:10.1038/289289a0.
- ^ D.L. Hawksworth and F. Rose(1976) Lichens as pollution monitors. Edward Arnold, Institute of Biology Series, No. 66. 60pp. ISBN 0713125543: 0713125551(pbk.)
- ^ R. Honegger (1988) Mycobionts. Chapter 3 in T.H. Nash (ed.) (1996) Lichen Biology. Cambridge University Press. ISBN 0521453682
- ^ T.H. Nash (editor) (1996) Lichen Biology. Cambridge University Press. ISBN 0521453682
- ^ Sciencemag.org
- ^ a b c d Honegger R. (2000). "Simon Schwender (1829–1919) and the dual hypothesis in lichens". Bryologist 103 (2): 307–13. doi:10.1639/0007-2745(2000)103[0307:SSATDH]2.0.CO;2. ISSN 0007-2745. JSTOR 3244159.
- ^ a b Kirk et al., pp. 378–81.
- ^ Friedl T, Büdel B.. "Photobionts". In Nash III TH. Lichen Biology. Cambridge: Cambridge University Press.
- ^ a b c Rikkinen J. (1995). "What's behind the pretty colors? A study on the photobiology of lichens". Bryobrothera 4: 1–226.
- ^ Smith, A.L. (1929). Lichens. Cambridge Botanical Handbooks. Cambridge University Press. ISBN 091642233X.
- ^ a b Büdel, B.; Scheidegger, C. (1996). "Thallus morphology and anatomy". Lichen Biology: 37–64.
- ^ "BBC NEWS | UK | Scotland | Insight into sex life of lichens". BBC News. 2006-09-18. http://news.bbc.co.uk/2/hi/uk_news/scotland/5356368.stm. Retrieved 2010-02-16.
- ^ Eichorn, Susan E., Evert, Ray F., and Raven, Peter H. 2005. Biology of Plants. New York (NY):W.H. Freeman and Company. 289 p.1.
- ^ Cook, Rebecca and McFarland, Kenneth. 1995. General Botany 111 Laboratory Manual. Knoxville (TN): University of Tennessee. 104 p.
- ^ Beschel RE (1950). "Flecten als altersmasstab Rezenter morainen". Zeitschrift für Gletscherkunde und Glazialgeologie 1: 152–161.
- ^ Oksanen I., I (2006). "Ecological and biotechnological aspects of lichens". Applied Microbiology and Biotechnology 73 (4): 723–34. doi:10.1007/s00253-006-0611-3. PMID 17082931.
- ^ "ESA — Human Spaceflight and Exploration - Lichen survives in space". http://www.esa.int/esaHS/SEMUJM638FE_index_0.html. Retrieved 2010-02-16.
- ^ Sancho, L.G.; De La Torre, R.; Horneck, G.; Ascaso, C.; De Los Rios, A.; Pintado, A.; Wierzchos, J.; Schuster, M. (2007). "Lichens survive in space: results from the 2005 LICHENS experiment". Astrobiology 7 (3): 443–54. Bibcode 2007AsBio...7..443S. doi:10.1089/ast.2006.0046. PMID 17630840.
- ^ a b c Nash TH. (2008). Lichen Biology (2nd ed.). Cambridge, UK: Cambridge University Press. pp. 299–314. ISBN 0-521-69216-4.
- ^ Knops JMH, Nash TH. (1991). "Mineral cycling and epiphytic lichens: Implications at the ecosystem level". Lichenologist 23 (03): 309–21. doi:10.1017/S0024282991000452.
- ^ Halonen P, Hyvarinen M, Kauppi M. (1993). "Emission related and repeated monitoring of element concentrations in the epiphytic lichen Hypogymnia physodes in a coastal area, western Finland". Annales Botanici Fennici 30: 251–61.
- ^ C.Michael Hogan. 2010. Abiotic factor. Encyclopedia of Earth. eds Emily Monosson and C. Cleveland. National Council for Science and the Environment. Washington DC
- ^ Beltman IH, de Kok LJ, Kuiper PJC, van Hasselt PR. (1980). "Fatty acid composition and chlorophyll content of epiphytic lichens and a possible relation to their sensitivity to air pollution". Oikos 35 (3): 321–26. doi:10.2307/3544647. JSTOR 3544647.
- ^ Speer BR, Waggoner B.. "Fossil Record of Lichens". University of California Museum of Paleontology. http://www.ucmp.berkeley.edu/fungi/lichens/lichenfr.html. Retrieved 2010-02-16.
- ^ Taylor, T.N.; Hass, H.; Remy, W.; Kerp, H. (1995). "The oldest fossil lichen". Nature 378 (6554): 244–244. Bibcode 1995Natur.378..244T. doi:10.1038/378244a0. http://www.uni-muenster.de/GeoPalaeontologie/Palaeo/Palbot/nature.html.
- ^ Taylor WA, Free CB, Helgemo R, Ochoada J. (2004). "SEM analysis of spongiophyton interpreted as a fossil lichen". International Journal of Plant Science 165 (5): 875–81. doi:10.1086/422129.
- ^ Jahren, A.H.; Porter, S.; Kuglitsch, J.J. (2003). "Lichen metabolism identified in Early Devonian terrestrial organisms". Geology 31 (2): 99–102. Bibcode 2003Geo....31...99J. doi:10.1130/0091-7613(2003)031<0099:LMIIED>2.0.CO;2. ISSN 0091-7613.
- ^ Fletcher, B.J.; Beerling, D.J.; Chaloner, W.G. (2004). "Stable carbon isotopes and the metabolism of the terrestrial Devonian organism Spongiophyton". Geobiology 2 (2): 107–119. doi:10.1111/j.1472-4677.2004.00026.x.
- ^ a b Retallack GJ. (2007). "Growth, decay and burial compaction of Dickinsonia, an iconic Ediacaran fossil" (PDF). Alcheringa: an Australasian Journal of Palaeontology 31 (3): 215–240. doi:10.1080/03115510701484705. http://www.informaworld.com/index/781217204.pdf. Retrieved 2008–02–04.
- ^ Retallack GJ. (1994). "Were the Ediacaran Fossils Lichens?". Paleobiology 20 (4): 523–44. ISSN 0094-8373. JSTOR 2401233.
- ^ Yuan X, Xiao S, Taylor TN. (2005). "Lichen-like symbiosis 600 million years ago". Science 308 (5724): 1017–20. Bibcode 2005Sci...308.1017Y. doi:10.1126/science.1111347. PMID 15890881.
- ^ Taylor TN.; Hass, Hagen; Kerp, Hans (1997). "A cyanolichens from the Lower Devnian Rhynie chert". American Journal of Botany 84 (7): 992–1004. doi:10.2307/2446290. JSTOR 2446290.
- ^ Poinar Jr., GO. (1992). Life in Amber. Standford University Press.
- ^ Peterson EB. (2000). "An overlooked fossil lichen (Lobariaceae)". Lichenologist 32 (3): 298–300. doi:10.1006/lich.1999.0257.
- ^ Gargas A, DePriest PT, Grube M, Tehler A. (1995). "Multiple origins of lichen symbioses in fungi suggested by SSU rDNA phylogeny". Science 268 (5216): 1492–95. Bibcode 1995Sci...268.1492G. doi:10.1126/science.7770775. PMID 7770775.
- ^ Honegger R. (1998). "The lichen symbiosis - what is so spectacular about it?". Lichenologist 30: 193–212.
- ^ Wedin M, Döring H, Gilenstam G. (2004). "Saprotrophy and lichenization as options for the same fungl species on different substrata: environmental plasticity and fungal lifestyles in the Strictis-Conotrema complex". New Phytologist 16: 4459–65.
- ^ Karatygin IV, Snigirevskaya NS, Vikulin SV., I. V.; Snigirevskaya, N. S.; Vikulin, S. V. (2009). "The most ancient terrestrial lichen Winfrenatia reticulata : A new find and new interpretation". Paleontological Journal 43 (1): 107–14. doi:10.1134/S0031030109010110.
- ^ Karatygin IV, Snigirevskaya NS, Vikulin SV. (2007). "Two types of symbiosis with participation of Fungi from Early Devonian Ecosystems". XV Congress of European Mycologists, Saint Petersburg, Russia, September 16–21, 2007. 1 (1): 226. http://vimeo.com/20073764.
- ^ Schoch CL, Sung G-H, López-Giráldez F et al., C. L.; Sung, G.-H.; Lopez-Giraldez, F.; Townsend, J. P.; Miadlikowska, J.; Hofstetter, V.; Robbertse, B.; Matheny, P. B. et al. (2009). "The Ascomycota tree of life: A phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits". Systematic Biology 58 (2): 224–39. doi:10.1093/sysbio/syp020. PMID 20525580.
- ^ Lutzoni et al.; Kauff, F.; Cox, C. J.; McLaughlin, D.; Celio, G.; Dentinger, B.; Padamsee, M.; Hibbett, D. et al. (2004). "Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits". American Journal of Botany 91 (10): 1446–80. doi:10.3732/ajb.91.10.1446.
- ^ Emmerich R, Giez I, Lange OL, Proksch P. (1993). "Toxicity and antifeedant activity of lichen compounds against the polyphagous herbivorous insect Spodoptera littoralis". Phytochemistry 33 (6): 1389–94. doi:10.1016/0031-9422(93)85097-B.
- ^ Casselman, Karen Leigh; Dean, Jenny (1999). Wild color: [the complete guide to making and using natural dyes]. New York: Watson-Guptill Publications. ISBN 0-8230-5727-5.
- ^ Hassuni I, Razxouk H. (2005). "Olivetol: Constituent of lichen Evernia prunastri Ach. or "oakmoss"". Physical and chemical News 26: 98–103. ISSN 1114-3800.
- ^ Themodelrailroader.com
- ^ Johnson, Christopher; James P. Bennett, Steven M. Biro, Juan Camilo Duque-Velasquez, Cynthia M. Rodriguez, Richard A. Bessen, Tonie E. Rocke (17th). "Degradation of the Disease-Associated Prion Protein by a Serine Protease from Lichens". PLoS one. doi:10.1371/journal.pone.0019836.
- ^ Yam, Philip. "Natural Born Prion Killers: Lichens Degrade "Mad Cow" Related Brain Pathogen". Scientific American. http://www.scientificamerican.com/blog/post.cfm?id=natural-born-prion-killers-lichens-2011-05-19. Retrieved 20 May 2011.
- ^ This was scraped from a dry, concrete-paved section of a drainage ditch. This entire image covers a square that is approximately 1.7 millimeters on a side. The numbered ticks on the scale represent distances of 230 micrometers, or slightly less than ¼ of a millimeter.
Bibliography
- Ahmadjian V. (1993). The Lichen Symbiosis. New York: John Wiley & Sons. ISBN 0-471-57885-1.
- Brodo, I.M., S.D. Sharnoff, and S. Sharnoff, 2001. Lichens of North America. Yale University Press, New Haven.
- Gilbert, O. 2004. The Lichen Hunters. The Book Guild Ltd. England.
- Haugan, Reidar / Timdal, Einar (1992): Squamarina scopulorum (Lecanoraceae), a new lichen species from Norway. Nordic Journal of Botany 12(3): 357-360.
- Hawksworth, D.L. and Seaward, M.R.D. 1977. Lichenology in the British Isles 1568 - 1975. The Richmond Publishing Co. Ltd., 1977.
- Kershaw, K.A. Physiological Ecology of Lichens, 1985. Cambridge University Press Cambridge.
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. (2008). Dictionary of the Fungi. (10th ed.). Wallingford: CABI. ISBN 9780851998268.
- Knowles M.C. (1929). "The Lichens of Ireland". Proceedings of the Royal Irish Academy 38: 1–32.
- Purvis, O.W., Coppins, B.J., Hawksworth, D.L., James, P.W. and Moore, D.M. (Editors) 1992. The Lichen Flora of Great Britain and Ireland. Natural History Museum, London.
- Sanders W.B. (2001). "Lichens: interface between mycology and plant morphology". Bioscience 51 (12): 1025–1035. doi:10.1641/0006-3568(2001)051[1025:LTIBMA]2.0.CO;2.
- Seaward M.R.D. (1984). "Census Catalogue of Irish Lichens". Glasra 8: 1–32.
External links
- LIAS light: an online interactive identification key for the lichen species of the World
- University of Sydney lichen biology
- Memorial University's NL Nature project, focusing primarily on lichens
- ESA article on lichen survivability in low earth orbit
- The British Lichen Society
- Lichens of North America
- Lichen Information and Pictures at blackturtle.us
- Pacific Northwest Fungi Online Journal, includes articles on lichens
- Fungi that discovered agriculture
- Pictures of Tropical Lichens
- High Resolution 1.5 Gigapixel Image of a Lichen Covered Rock
- Good example of a Lichen
 "Lichens". New International Encyclopedia. 1905.
"Lichens". New International Encyclopedia. 1905. "Lichens". Encyclopedia Americana. 1920.
"Lichens". Encyclopedia Americana. 1920.
Categories:- Lichens
- Bioindicators
- Cryptogams
- Polyextremophiles
- Oligotrophs
- Indicator species
- Mycology
- Symbiosis
Wikimedia Foundation. 2010.