- Tetrahydrocannabinol
-
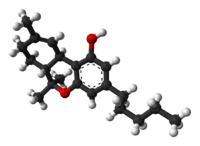
Tetrahydrocannabinol (THC) 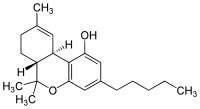
Systematic (IUPAC) name (−)-(6aR,10aR)-6,6,9-trimethyl-
3-pentyl-6a,7,8,10a-tetrahydro-
6H-benzo[c]chromen-1-olClinical data Pregnancy cat. C Legal status Schedule I and III (US) Dependence liability Very Low Routes Orally Pharmacokinetic data Bioavailability 10-35% (inhalation), 6-20% (oral)[1] Protein binding 95-99%[1] Metabolism mostly hepatic by CYP2C[1] Half-life 1.6-59 hours,[1] 25-36 hours (orally administered Dronabinol) Excretion 65-80% (feces), 20-35% (urine) as acid metabolites[1] Identifiers CAS number 1972-08-3 
ATC code A04AD10 PubChem CID 16078 DrugBank DB00470 ChemSpider 15266 
UNII 7J8897W37S 
ChEMBL CHEMBL465 
Synonyms Dronabinol Chemical data Formula C21H30O2 Mol. mass 314.45 SMILES eMolecules & PubChem Physical data Boiling point 157 °C (315 °F) [3] Solubility in water 0.0028[2] (23 °C) mg/mL (20 °C) Spec. rot -152° (ethanol)  (what is this?) (verify)
(what is this?) (verify)Tetrahydrocannabinol (
 /ˌtɛtrəˌhaɪdrɵkəˈnæbɨnɒl/ tet-rə-hy-drə-kə-nab-i-nol; THC), also known as delta-9-tetrahydrocannabinol (Δ9-THC), Δ1-THC (using an older chemical nomenclature), or dronabinol, is the main psychoactive substance found in the cannabis plant. It was first isolated in 1964.[4][5][6] In pure form, it is a glassy solid when cold, and becomes viscous and sticky if warmed. An aromatic terpenoid, THC has a very low solubility in water, but good solubility in most organic solvents.
/ˌtɛtrəˌhaɪdrɵkəˈnæbɨnɒl/ tet-rə-hy-drə-kə-nab-i-nol; THC), also known as delta-9-tetrahydrocannabinol (Δ9-THC), Δ1-THC (using an older chemical nomenclature), or dronabinol, is the main psychoactive substance found in the cannabis plant. It was first isolated in 1964.[4][5][6] In pure form, it is a glassy solid when cold, and becomes viscous and sticky if warmed. An aromatic terpenoid, THC has a very low solubility in water, but good solubility in most organic solvents.Like most pharmacologically-active secondary metabolites of plants, THC in cannabis is assumed to be involved in self-defense, perhaps against herbivores.[7] THC also possesses high UV-B (280-315 nm) absorption properties, which, it has been speculated, could protect the plant from harmful UV radiation exposure.[8][9][10]
Dronabinol is the International Nonproprietary Name (INN) for a pure isomer of THC, (–)-trans-Δ9-tetrahydrocannabinol, which is the main isomer in cannabis.[11] It is sold as Marinol (a registered trademark of Solvay Pharmaceuticals). Dronabinol is also marketed, sold, and distributed by PAR Pharmaceutical Companies under the terms of a license and distribution agreement with SVC pharma LP, an affiliate of Rhodes Technologies.
Contents
Pharmacology
The pharmacological actions of THC result from its binding to the cannabinoid receptor CB1, located mainly in the central nervous system, and the CB2 receptor, mainly present in cells of the immune system. It acts as a partial agonist on both receptors, i.e., it activates them but not to their full extent. The psychoactive effects of THC are mediated by its activation of the CB1 receptor, which is the most abundant G protein-coupled receptor in the brain.[citation needed]
The presence of these specialized receptors in the brain implied to researchers that endogenous cannabinoids are manufactured by the body, so the search began for a substance normally manufactured in the brain that binds to these receptors, the so-called natural ligand or agonist, leading to the eventual discovery of anandamide, 2-arachidonoyl glyceride (2-AG), and other related compounds known as endocannabinoids. This is similar to the story of the discovery of endogenous opiates (endorphins, enkephalins, and dynorphin), after the realization that morphine and other opiates bind to specific receptors in the brain. In addition, it has been shown that cannabinoids, through an unknown mechanism, activate endogenous opioid pathways involving the μ1 opioid receptor, precipitating a dopamine release in the nucleus accumbens. The effects of the drug can be suppressed by the CB1 cannabinoid receptor antagonist rimonabant (SR141716A) as well as opioid receptor antagonists (opioid blockers) naloxone and naloxonazine.[12]
The mechanism of endocannabinoid synaptic transmission is thought to occur as follows: First, transmission of the excitatory neurotransmitter glutamate causes an influx of calcium ions into the post-synaptic neuron. Through a mechanism not yet fully understood, the presence of post-synaptic calcium induces the production of endocannabinoids in the post-synaptic neuron. These endocannabinoids (such as anandamide), then, are released into the synaptic cleft, where binding occurs at cannabinoid receptors present on pre-synaptic neurons, where they modulate neurotransmission. Thus, this form of neurotransmission is termed retrograde transmission, as the signal is carried in the opposite direction of orthodox propagation, which previously was thought to be exclusively one way.[13]
THC has mild to moderate analgesic effects, and cannabis can be used to treat pain. The mechanism for analgesic effects caused directly by THC or other cannabinoid agonists is not fully understood. Other effects include relaxation; euphoria; altered space-time perception; alteration of visual, auditory, and olfactory senses; loss of anxiety;[14][unreliable medical source?] anxiety in neurotic individuals or individuals unfamiliar with effects;[14] disorientation;[14] fatigue; and appetite stimulation (colloquially known as "the munchies"). The mechanism for appetite stimulation in subjects is believed to result from activity in the gastro-hypothalamic axis.[citation needed] CB1 activity in the hunger centers in the hypothalamus increases the palatability of food when levels of a hunger hormone ghrelin increase prior to consuming a meal. After chyme is passed into the duodenum, signaling hormones such as cholecystokinin and leptin are released, causing reduction in gastric emptying and transmission of satiety signals to the hypothalamus. Cannabinoid activity is reduced through the satiety signals induced by leptin release. It also has anti-emetic properties, and also may reduce aggression in certain subjects.[citation needed]
THC has an active metabolite, 11-Hydroxy-THC, which may also play a role in the analgesic and recreational effects of cannabis.[citation needed]
The α7 nicotinic receptor antagonist methyllycaconitine can block self-administration of THC in rats comparable to the effects of varenicline on nicotine administration.[15]
Two studies indicate that THC also has an anticholinesterase action[16][17] which may implicate it as a potential treatment for Alzheimer's and Myasthenia Gravis.
Toxicity
See also: Health issues and effects of cannabisThere has never been a documented human fatality from overdosing on tetrahydrocannabinol or cannabis in its natural form.[18] However, the synthetic THC pill Marinol was cited by the FDA as being responsible for 4 of the 11,687 deaths from 17 different FDA approved drugs between January 1, 1997 to June 30, 2005.[19] Information about THC's toxicity is derived from animal studies. The toxicity depends on the route of administration and the laboratory animal. Absorption is limited by serum lipids, which can become saturated with THC, mitigating toxicity.[20] According to the Merck Index, 12th edition, THC has an LD50 (dose killing half of the research subjects) value of 1270 mg/kg (male rats) and 730 mg/kg (female rats) administered orally dissolved in sesame oil.[21] The LD50 value for rats by inhalation of THC is 42 mg/kg of body weight.[21]
Animal Administration LD50 [mg/kg] rat oral 666 [20] rat (male) oral 1270 [21] rat (female) oral 730 [21] rat inhalation 42 [21] rat intraperitoneal 373 [20] rat intravenous 29 [20] mouse intravenous 42 [20] mouse oral 482 [20] mouse intraperitoneal 168 [20] monkey (LDLo) intravenous 128 [20] dog oral 525 [20] Research
The discovery of THC was first described in "Isolation, structure and partial synthesis of an active constituent of hashish", published in the Journal of the American Chemical Society in 1964.[4] Research was also published in the academic journal Science, with "Marijuana chemistry" by Raphael Mechoulam in June 1970,[22] followed by "Chemical basis of hashish activity" in August 1970.[23] In the latter, the team of researchers from Hebrew University Pharmacy School and Tel Aviv University Medical School experimented on monkeys to isolate the active compounds in hashish. Their results provided evidence that, except for tetrahydrocannabinol, no other major active compounds were present in hashish.
Studies in humans
A number of studies show that THC provides medical benefits for cancer and AIDS patients by increasing appetite and decreasing nausea. It has also been shown to assist some glaucoma patients by reducing pressure within the eye, and is used in the form of cannabis by a number of multiple sclerosis patients, who use it to alleviate neuropathic pain and spasticity. The National Multiple Sclerosis Society is currently supporting further research into these uses.[24]
In August 2009 a phase IV clinical trial by the Hadassah Medical Center in Jerusalem, Israel started to investigate the effects of THC on post-traumatic stress disorders.[25] THC and other cannabinoid agonists have been shown to be beneficial both in open label studies, as well as in laboratory experiments with animals to ameliorate post-traumatic stress disorders.
Preliminary research on synthetic THC has been conducted on patients with Tourette syndrome, with results suggesting that it may help in reducing nervous tics and urges by a significant degree. Research on twelve patients showed that Marinol reduced tics with no significant adverse effects. A six-week controlled study on 24 patients showed that the patients taking dronabinol had a significant reduction in tic severity without serious adverse effects. More significant reduction in tic severity was reported with longer treatment. No detrimental effects on cognitive functioning and a trend towards improvement in cognitive functioning were reported during and after treatment.[citation needed]
Dronabinol's usefulness as a treatment for TS cannot be determined until/unless longer controlled studies on larger samples are undertaken.[26][27][28]
Research on THC has shown that Cannabinoid receptors are responsible for mediated inhibition of dopamine release in the retina.[29]
Studies in animals and in vitro
New scientific evidence is showing that THC can prevent Alzheimer's Disease in an animal model by preventing the inflammation caused by microglia cells which are activated by binding of amyloid protein.[30]
In in-vitro experiments, THC at extremely high concentrations, which could not be reached with commonly-consumed doses, caused inhibition of plaque formation (which are associated with Alzheimer's disease) better than currently-approved drugs.[31]
THC may also be an effective anti-cancer treatment, with studies showing tumor size reduction in mice conducted in 1975[32] and 2007,[33] as well as in a pilot study in humans with glioblastoma multiforme (a type of brain cancer).[34] THC has also been found to attenuate conditioned retching and sickness, experimentally verifying anecdotal reports that THC alleviates nausea and vomiting when undergoing chemotherapy.[35]
A two-year study in which rats and mice were force-fed tetrahydrocannabinol dissolved in corn oil showed reduced body mass, enhanced survival rates, and decreased tumor incidences in several sites, mainly organs under hormonal control. It also caused testicular atrophy and uterine and ovarian hypoplasia, as well as hyperactivity and convulsions immediately after administration, of which the onset and frequency were dose related.[36]
Research in rats indicates that THC prevents hydroperoxide-induced oxidative damage as well as or better than other antioxidants in a chemical (Fenton reaction) system and neuronal cultures.[37] In mice low doses of Δ9-THC reduces the progression of atherosclerosis.[38]
Research has also shown that past claims of brain damage from cannabis use fail to hold up to the scientific method.[39] Instead, recent studies with synthetic cannabinoids show that activation of CB1 receptors can facilitate neurogenesis,[40] as well as neuroprotection,[41] and can even help prevent natural neural degradation from neurodegenerative diseases such as MS, Parkinson's, and Alzheimer's. This, along with research into the CB2 receptor (throughout the immune system), has given the case for medical marijuana more support.[42][43] THC is both a CB1 and CB2 agonist.[44]
Research indicating negative side-effects
Conceivable long-term ill effects of THC on humans are disputed, yet its status as an illegal drug in most countries makes research difficult.[citation needed]
Some studies claim a variety of negative effects associated with long-term use, including short-term memory loss.[45][46] Some studies have found little or no difference in MRI scans between user groups and non-using control groups[citation needed]. Using positron emission tomography (PET), one study reports altered memory-related brain function (23% better memory for the marijuana users in recalling the end of a list of things to remember, but 19% worse memory for marijuana users in recalling the middle of a list of things to remember) in chronic daily marijuana users.[47]
Some studies have suggested that cannabis users have a greater risk of developing psychosis than non-users. This risk is most pronounced in cases with an existing risk of psychotic disorder.[48] Other studies have made similar associations, especially in individuals predisposed to psychosis prior to cannabis use.[49] A 2005 paper from the Dunedin study suggested an increased risk in the development of psychosis linked to polymorphisms in the COMT gene.[50] However, a more recent study cast doubt on the proposed connection between this gene and the effects of cannabis on the development of psychosis.[51] A literature review on the subject concluded that "Cannabis use appears to be neither a sufficient nor a necessary cause for psychosis. It is a component cause, part of a complex constellation of factors leading to psychosis."[52] Likewise, a French review from 2009 came to a conclusion that cannabis use, particularly that before age 15, was a factor in the development of schizophrenic disorders.[53]
A 2008 German review reported that cannabis was a causal factor in some cases of schizophrenia and stressed the need for better education among the public due to increasingly relaxed access to cannabis.[54] Though cannabis use has increased dramatically in several countries over the past few decades, the rates of psychosis and schizophrenia have not generally increased, casting some doubt over whether the drug can cause cases that would not otherwise have occurred.[55]
Research from 2007 reported a correlation between cannabis use and increased cognitive function in schizophrenic patients.[56]
A 2008 National Institutes of Health study of 18 chronic heavy marijuana users with cardiac and cerebral abnormalities (averaging 28g to 272g (1 to 8 oz) weekly) and 24 controls found elevated levels of apolipoprotein C-III (apoC-III) in the chronic smokers.[57] An increase in apoC-III levels induces the development of hypertriglyceridemia.
A 2008 study by the University of Melbourne of 15 heavy marijuana users (consuming at least 5 marijuana cigarettes daily for on average 20 years) and 16 controls found an average size difference for the smokers in the hippocampus (12 percent smaller) and the amygdala (7 percent smaller).[58] It has been suggested that such effects can be reversed with long term abstinence.[59] However, the study indicates that they are unsure that the problems were caused by marijuana alone. Furthermore, this correlation might suggest self-medication by individuals with these brain features.
A 2008 study at Karolinska Institute suggested that young rats treated with THC received an increased motivation for drug use, heroin in the study, under conditions of stress.[60][61]
A 2009 study found that there was a high prevalence of cannabis in the toxicologic analysis of homicide (22%) and suicide victims (11%) in Australia.[62] In a similar study from Sweden it was also found that suicide victims had a significant higher use of cannabis, but the authors found that "this was explained by markers of psychological and behavioural problems."[63]
Biosynthesis
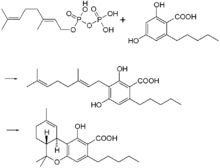
In the cannabis plant, THC occurs mainly as tetrahydrocannabinol carboxylic acid (THC-COOH). Geranyl pyrophosphate and olivetolic acid react, catalysed by an enzyme to produce cannabigerolic acid,[64] which is cyclized by the enzyme THC acid synthase to give THC-COOH. Over time, or when heated, THC-COOH is decarboxylated producing THC. The pathway for THC-COOH biosynthesis is similar to that which produces the bitter acid humulone in hops.[65]
Metabolism
THC is metabolized mainly to 11-OH-THC (11-hydroxy-THC) by the human body. This metabolite is still psychoactive and is further oxidized to 11-Nor-9-carboxy-THC (THC-COOH). In humans and animals, more than 100 metabolites could be identified, but 11-OH-THC and THC-COOH are the dominating metabolites. Metabolism occurs mainly in the liver by cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP3A4. More than 55% of THC is excreted in the feces and ~20% in the urine. The main metabolite in urine is the ester of glucuronic acid and THC-COOH and free THC-COOH. In the feces, mainly 11-OH-THC was detected.[66]
Detection in body fluids
THC, 11-OH-THC and THC-COOH can be detected and quantitated in blood, urine, hair, oral fluid or sweat using a combination of immunoassay and chromatographic techniques as part of a drug use testing program or in a forensic investigation of a traffic or other criminal offense or suspicious death. The concentrations obtained from such analyses can often be helpful in distinguishing active from passive use or prescription from illicit use, the route of administration (oral versus smoking), elapsed time since use and extent or duration of use.[67][68][69]
Dronabinol
Synthesized THC is known as dronabinol. It is available as a prescription drug (under Marinol[70]) in several countries including the United States and Germany. In the United States, Marinol is a Schedule III drug, available by prescription, considered to be non-narcotic and to have a low risk of physical or mental dependence. Efforts to get cannabis rescheduled as analogous to Marinol have not succeeded thus far, though a 2002 petition has been accepted by the DEA. As a result of the rescheduling of Marinol from Schedule II to Schedule III, refills are now permitted for this substance. Marinol has been approved by the U.S. Food and Drug Administration (FDA) in the treatment of anorexia in AIDS patients, as well as for refractory nausea and vomiting of patients undergoing chemotherapy, which has raised much controversy as to why natural THC is still a schedule I drug.[71]
An analog of dronabinol, nabilone, is available commercially in Canada under the trade name Cesamet, manufactured by Valeant Pharmaceuticals. Cesamet has also received FDA approval and began marketing in the U.S. in 2006; it is a Schedule II drug.[citation needed]
In April 2005, Canadian authorities approved the marketing of Sativex, a mouth spray for multiple sclerosis patients, who can use it to alleviate neuropathic pain and spasticity. Sativex contains tetrahydrocannabinol together with cannabidiol. It is marketed in Canada by GW Pharmaceuticals, being the first cannabis-based prescription drug in the world (in modern times). In addition, Sativex received European regulatory approval in 2010.[citation needed]
Comparisons to medical marijuana
Main article: Medical marijuanaFemale cannabis plants contain more than 60 cannabinoids, including cannabidiol (CBD), thought to be the major anticonvulsant that helps multiple sclerosis patients;[72] and cannabichromene (CBC), an anti-inflammatory which may contribute to the pain-killing effect of cannabis.[73]
It takes over one hour for Marinol to reach full systemic effect,[74] compared to minutes for smoked or vaporized cannabis.[75] Some patients accustomed to inhaling just enough cannabis smoke to manage symptoms have complained of too-intense intoxication from Marinol's predetermined dosages. Many patients have said that Marinol produces a more acute psychedelic effect than cannabis, and it has been speculated that this disparity can be explained by the moderating effect of the many non-THC cannabinoids present in cannabis. For that reason, alternative THC-containing medications based on botanical extracts of the cannabis plant such as nabiximols are being developed. Mark Kleiman, director of the Drug Policy Analysis Program at UCLA's School of Public Affairs said of Marinol, "It wasn't any fun and made the user feel bad, so it could be approved without any fear that it would penetrate the recreational market, and then used as a club with which to beat back the advocates of whole cannabis as a medicine."[76] United States federal law currently registers dronabinol as a Schedule III controlled substance, but all other cannabinoids remain Schedule I, excepting synthetics like nabilone.[citation needed]
Regulatory history
Since at least 1986, the trend has been for THC in general, and especially the Marinol preparation, to be downgraded to less and less stringently-controlled schedules of controlled substances, in the U.S. and throughout the rest of the world.
On July 13, 1986, the Drug Enforcement Administration (DEA) issued a Final Rule and Statement of Policy authorizing the "Rescheduling of Synthetic Dronabinol in Sesame Oil and Encapsulated in Soft Gelatin Capsules From Schedule I to Schedule II" (DEA 51 FR 17476-78). This permitted medical use of Marinol, albeit with the severe restrictions associated with Schedule II status. For instance, refills of Marinol prescriptions were not permitted. At its 1045th meeting, on April 29, 1991, the Commission on Narcotic Drugs, in accordance with article 2, paragraphs 5 and 6, of the Convention on Psychotropic Substances, decided that Δ9-tetrahydrocannabinol (also referred to as Δ9-THC) and its stereochemical variants should be transferred from Schedule I to Schedule II of that Convention. This released Marinol from the restrictions imposed by Article 7 of the Convention (See also United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances).[citation needed]
An article published in the April–June 1998 issue of the Journal of Psychoactive Drugs found that "Healthcare professionals have detected no indication of scrip-chasing or doctor-shopping among the patients for whom they have prescribed dronabinol". The authors state that Marinol has a low potential for abuse.[77]
In 1999, Marinol was rescheduled from Schedule II to III of the Controlled Substances Act, reflecting a finding that THC had a potential for abuse less than that of cocaine, and heroin. This rescheduling comprised part of the argument for a 2002 petition for removal of cannabis from Schedule I of the Controlled Substances Act, in which petitioner Jon Gettman noted, "Cannabis is a natural source of dronabinol (THC), the ingredient of Marinol, a Schedule III drug. There are no grounds to schedule cannabis in a more restrictive schedule than Marinol".[78]
At its 33rd meeting, the World Health Organization Expert Committee on Drug Dependence recommended transferring THC to Schedule IV of the Convention, citing its medical uses and low abuse potential.[citation needed]
See also
- Cannabis (drug)
- Psychoactive drug
- Cannabinoids
- Anandamide, 2-Arachidonoylglycerol, endogenous cannabinoid agonists
- Cannabidiol (CBD), an isomer of THC
- Cannabinol (CBN), a metabolite of THC
- HU-210, WIN 55,212-2, JWH-133, synthetic cannabinoid agonists
- Medical cannabis
- War on Drugs
- Cannabis rescheduling in the United States
- Health issues and the effects of cannabis
References
- Notes
- ^ a b c d e Grotenhermen F (2003). "Pharmacokinetics and pharmacodynamics of cannabinoids". Clin Pharmacokinet 42 (4): 327–60. doi:10.2165/00003088-200342040-00003. PMID 12648025.
- ^ Garrett, Edward R.; C. Anthony Hunt (July 1974). "Physicochemical properties, solubility, and protein binding of Δ9 -tetrahydrocannabinol". J. Pharm. Sci. 63 (7): 1056–1064. doi:10.1002/jps.2600630705. PMID 4853640.
- ^ "Cannabis and Cannabis Extracts: Greater Than the Sum of Their Parts?". www.haworthpress.com. http://www.omma1998.org/McPartland-Russo-JCANT%201(3-4)-2001.pdf. Retrieved 2011-01-25.
- ^ a b Gaoni, Y.; Mechoulam, R. (1964). Journal of the American Chemical Society 86 (8): 1646–1647. doi:10.1021/ja01062a046.
- ^ Interview with the winner of the first ECNP Lifetime Achievement Award: Raphael Mechoulam, Israel February 2007
- ^ Geller, Tom (2007). "Cannabinoids: A Secret History". Chemical Heritage Newsmagazine 25 (2). Archived from the original on June 19, 2008. http://web.archive.org/web/20080619013348/http://chemicalheritage.org/pubs/ch-v25n2-articles/feature_cannabinoids.html.
- ^ Pate, David W. (1994). "Chemical ecology of Cannabis". Journal of the International Hemp Association 1 (29): 32–37. http://www.kew.org/kbd/detailedresult.do?id=91816.
- ^ Pate, David W. (1983). "Possible role of ultraviolet radiation in evolution ofCannabis chemotypes". Economic Botany 37 (4): 396–405. doi:10.1007/BF02904200.
- ^ Lydon, John; Teramura, Alan H. (1987). "Photochemical decomposition of cannabidiol in its resin base". Phytochemistry 26 (4): 1216–1217. doi:10.1016/S0031-9422(00)82388-2.
- ^ Lydon, John; Teramura, Alan H.; Coffman, C. Benjamin (1987). "UV-B RADIATION EFFECTS ON PHOTOSYNTHESIS, GROWTH and CANNABINOID PRODUCTION OF TWO Cannabis sativa CHEMOTYPES". Photochemistry and Photobiology 46 (2): 201–206. doi:10.1111/j.1751-1097.1987.tb04757.x. PMID 3628508.
- ^ "List of psychotropic substances under international control" (PDF). http://www.incb.org/pdf/e/list/green.pdf. Retrieved 2011-04-20.[page needed]
- ^ Lupica, Carl R; Riegel, Arthur C; Hoffman, Alexander F (2004). "Marijuana and cannabinoid regulation of brain reward circuits". British Journal of Pharmacology 143 (2): 227–34. doi:10.1038/sj.bjp.0705931. PMC 1575338. PMID 15313883. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1575338.
- ^ Vaughan, C.W. & Christie, M.J. (2005). "Retrograde Signalling by Endocannabinoids". In Abood, Mary Ellen & Pertwee, Roger G.. Cannabinoids. Birkhäuser. ISBN 9783540225652. http://books.google.com/books?id=YbBYBT1HZgYC&pg=PA367.
- ^ a b c "Medical Marijuana Reports". National Organization for the Reform of Marijuana Laws. April 17, 2011. http://norml.org/index.cfm?Group_ID=3472. Retrieved 2011-04-20.
- ^ Solinas, M.; Scherma, M.; Fattore, L.; Stroik, J.; Wertheim, C.; Tanda, G.; Fratta, W.; Goldberg, S. R. (2007). "Nicotinic 7 Receptors as a New Target for Treatment of Cannabis Abuse". Journal of Neuroscience 27 (21): 5615–20. doi:10.1523/JNEUROSCI.0027-07.2007. PMID 17522306. Lay summary – New Scientist (22 May 2007).
- ^ Brown, Hugh (1972). "Possible anticholinesterase-like effects of trans(−)δ8 and -δ9tetrahydrocannabinol as observed in the general motor activity of mice". Psychopharmacologia 27 (2): 111–6. doi:10.1007/BF00439369. PMID 4638205.
- ^ Eubanks, Lisa M.; Rogers, Claude J.; Beuscher, 4th; Koob, George F.; Olson, Arthur J.; Dickerson, Tobin J.; Janda, Kim D. (2006). "A Molecular Link Between the Active Component of Marijuana and Alzheimer's Disease Pathology". Molecular Pharmaceutics 3 (6): 773–7. doi:10.1021/mp060066m. PMC 2562334. PMID 17140265. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2562334.
- ^ Walker, J.Michael; Huang, Susan M (2002). "Cannabinoid analgesia". Pharmacology & Therapeutics 95 (2): 127–35. doi:10.1016/S0163-7258(02)00252-8. "…to date, there are no deaths known to have resulted from overdose of cannabis. (p. 128)"
- ^ "Deaths from Marijuana v. 17 FDA-Approved Drugs" (PDF). 2005-06-30. http://www.oregon.gov/Pharmacy/Imports/Marijuana/Public/DeathsFromMarijuanaV17FDAdrugs.pdf. Retrieved 2011-02-03.
- ^ a b c d e f g h i "Erowid Cannabis Vault : THC Material Safety Data Sheet". Erowid.org. http://www.erowid.org/plants/cannabis/thc_data_sheet.shtml. Retrieved 2011-04-20.[unreliable medical source?]
- ^ a b c d e Erowid. "Cannabis Chemistry". http://www.erowid.org/plants/cannabis/cannabis_chemistry.shtml. Retrieved 2006-03-20.[unreliable medical source?]
- ^ Mechoulam, R. (1970). "Marihuana Chemistry". Science 168 (3936): 1159–1165. doi:10.1126/science.168.3936.1159.
- ^ Mechoulam, R.; Shani, A.; Edery, H.; Grunfeld, Y. (1970). "Chemical Basis of Hashish Activity". Science 169 (3945): 611–612. doi:10.1126/science.169.3945.611.
- ^ "Marijuana (Cannabis)". National Multiple Sclerosis Society. http://www.nationalmssociety.org/about-multiple-sclerosis/treatments/complementary--alternative-medicine/marijuana/index.aspx. Retrieved 2009-09-05.
- ^ ClinicalTrials.gov NCT00965809 Add on Study on Δ9-THC Treatment for Posttraumatic Stress Disorders (PTSD) (THC_PTSD)
- ^ Müller-Vahl, K. R.; Schneider, U.; Koblenz, A.; Jöbges, M.; Kolbe, H.; Daldrup, T.; Emrich, H. M. (2002). "Treatment of Tourette's Syndrome with Δ9-Tetrahydrocannabinol (THC): A Randomized Crossover Trial". Pharmacopsychiatry 35 (2): 57–61. doi:10.1055/s-2002-25028. PMID 11951146.
- ^ Muller-Vahl, Kirsten R.; Schneider, Udo; Prevedel, Heidrun; Theloe, Karen; Kolbe, Hans; Daldrup, Thomas; Emrich, Hinderk M. (2003). "Delta 9-Tetrahydrocannabinol (THC) is Effective in the Treatment of Tics in Tourette Syndrome". The Journal of Clinical Psychiatry 64 (4): 459–65. doi:10.4088/JCP.v64n0417. PMID 12716250.
- ^ Müller-Vahl, Kirsten R; Prevedel, Heidrun; Theloe, Karen; Kolbe, Hans; Emrich, Hinderk M; Schneider, Udo (2003). "Treatment of Tourette Syndrome with Delta-9-Tetrahydrocannabinol (Δ9-THC): No Influence on Neuropsychological Performance". Neuropsychopharmacology 28 (2): 384–8. doi:10.1038/sj.npp.1300047. PMID 12589392.
- ^ Schlicker, E; Timm, J; Göthert, M (1996). "Cannabinoid receptor-mediated inhibition of dopamine release in the retina". Naunyn-Schmiedeberg's archives of pharmacology 354 (6): 791–5. doi:10.1007/BF00166907. PMID 8971741.
- ^ Ramirez, B. G.; Blázquez, C; Gómez Del Pulgar, T; Guzmán, M; De Ceballos, ML (2005). "Prevention of Alzheimer's Disease Pathology by Cannabinoids: Neuroprotection Mediated by Blockade of Microglial Activation". Journal of Neuroscience 25 (8): 1904–13. doi:10.1523/JNEUROSCI.4540-04.2005. PMID 15728830.
- ^ Eubanks, Lisa M.; Rogers, Claude J.; Beuscher, 4th; Koob, George F.; Olson, Arthur J.; Dickerson, Tobin J.; Janda, Kim D. (2006). "A Molecular Link Between the Active Component of Marijuana and Alzheimer's Disease Pathology". Molecular Pharmaceutics 3 (6): 773–7. doi:10.1021/mp060066m. PMC 2562334. PMID 17140265. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2562334.
- ^ Munson, AE; Harris, LS; Friedman, MA; Dewey, WL; Carchman, RA (1975). "Antineoplastic activity of cannabinoids". Journal of the National Cancer Institute 55 (3): 597–602. PMID 1159836.
- ^ Preet, A; Ganju, R K; Groopman, J E (2007). "Δ9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo". Oncogene 27 (3): 339–46. doi:10.1038/sj.onc.1210641. PMID 17621270.
- ^ Guzmán, M; Duarte, M J; Blázquez, C; Ravina, J; Rosa, M C; Galve-Roperh, I; Sánchez, C; Velasco, G et al. (2006). "A pilot clinical study of Δ9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme". British Journal of Cancer 95 (2): 197–203. doi:10.1038/sj.bjc.6603236. PMC 2360617. PMID 16804518. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2360617.
- ^ Kemp, Stephen W.P. (2001). The effect of detla-9-tetrahydrocannabinol (THC) on lithium-induced sickness reactions in both rats (Rattus norvegicus) and the house musk shrew (Suncus murinus) (M.A. thesis) Wilfrid Laurier University
- ^ Chan, P; Sills, RC; Braun, AG; Haseman, JK; Bucher, JR (1996). "Toxicity and Carcinogenicity of Δ9-Tetrahydrocannabinol in Fischer Rats and B6C3F1 Mice". Fundamental and Applied Toxicology 30 (1): 109–17. doi:10.1006/faat.1996.0048. PMID 8812248.
- ^ Hampson, A. J.; Grimaldi, M; Axelrod, J; Wink, D (1998). "Cannabidiol and (−)Δ9-tetrahydrocannabinol are neuroprotective antioxidants". Proceedings of the National Academy of Sciences 95 (14): 8268–73. Bibcode 1998PNAS...95.8268H. doi:10.1073/pnas.95.14.8268. PMC 20965. PMID 9653176. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=20965.
- ^ Steffens, Sabine; Veillard, Niels R.; Arnaud, Claire; Pelli, Graziano; Burger, Fabienne; Staub, Christian; Zimmer, Andreas; Frossard, Jean-Louis et al. (2005). "Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice". Nature 434 (7034): 782–6. doi:10.1038/nature03389. PMID 15815632.
- ^ Grant, Igor; Gonzalez, Raul; Carey, Catherine L.; Natarajan, Loki; Wolfson, Tanya (2003). "Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study". Journal of the International Neuropsychological Society 9 (5). doi:10.1017/S1355617703950016. Lay summary – WebMD (July 1, 2003).
- ^ Jiang, W.; Zhang, Y; Xiao, L; Van Cleemput, J; Ji, SP; Bai, G; Zhang, X (2005). "Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects". Journal of Clinical Investigation 115 (11): 3104–3116. doi:10.1172/JCI25509. PMC 1253627. PMID 16224541. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1253627.
- ^ Sarne, Yosef; Mechoulam, Raphael (2005). "Cannabinoids: Between Neuroprotection and Neurotoxicity". Current Drug Targets - CNS & Neurological Disorders 4 (6): 677–684. doi:10.2174/156800705774933005.
- ^ Correa, Fernando; Mestre, Leyre; Molina-Holgado, Eduardo; Arevalo-Martin, Angel; Docagne, Fabian; Romero, Eva; Molina-Holgado, Francisco; Borrell, Jose et al. (2005). "The Role of Cannabinoid System on Immune Modulation: Therapeutic Implications on CNS Inflammation". Mini Reviews in Medicinal Chemistry 5 (7): 671–675. doi:10.2174/1389557054368790. PMID 16026313.
- ^ Fernández-Ruiz, Javier; Romero, Julián; Velasco, Guillermo; Tolón, Rosa M.; Ramos, José A.; Guzmán, Manuel (2007). "Cannabinoid CB2 receptor: a new target for controlling neural cell survival?". Trends in Pharmacological Sciences 28 (1): 39–45. doi:10.1016/j.tips.2006.11.001. PMID 17141334.
- ^ Pertwee, Roger G (2010). "Cannabinoid Receptor Ligands". Tocris. http://www.tocris.com/pdfs/pdf_downloads/Cannabinoid_Receptor_Ligands_Review.pdf. Retrieved 2011-04-20.
- ^ Bartholomew, J.; Holroyd, S.; Heffernan, T. M (2009). "Does cannabis use affect prospective memory in young adults?". Journal of Psychopharmacology 24 (2): 241–6. doi:10.1177/0269881109106909. PMID 19825904.
- ^ Indlekofer, F; Piechatzek, M; Daamen, M; Glasmacher, C; Lieb, R; Pfister, H; Tucha, O; Lange, K. et al. (2008). "Reduced memory and attention performance in a population-based sample of young adults with a moderate lifetime use of cannabis, ecstasy and alcohol". Journal of Psychopharmacology 23 (5): 495–509. doi:10.1177/0269881108091076. PMID 18635709.
- ^ Block, R; O'Leary, Daniel S; Hichwa, Richard D; Augustinack, Jean C; Boles Ponto, Laura L; Ghoneim, M.M; Arndt, Stephan; Hurtig, Richard R et al. (2002). "Effects of frequent marijuana use on memory-related regional cerebral blood flow". Pharmacology Biochemistry and Behavior 72: 237–50. doi:10.1016/S0091-3057(01)00771-7.
- ^ Moore, Theresa HM; Zammit, Stanley; Lingford-Hughes, Anne; Barnes, Thomas RE; Jones, Peter B; Burke, Margaret; Lewis, Glyn (2007). "Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review". The Lancet 370 (9584): 319–28. doi:10.1016/S0140-6736(07)61162-3. PMID 17662880.
- ^ Henquet, C.; Krabbendam, L; Spauwen, J; Kaplan, C; Lieb, R; Wittchen, HU; Van Os, J (2005). "Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people". BMJ 330 (7481): 11–0. doi:10.1136/bmj.38267.664086.63. PMC 539839. PMID 15574485. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=539839.
- ^ Caspi, A; Moffitt, T; Cannon, M; McClay, J; Murray, R; Harrington, H; Taylor, A; Arseneault, L et al. (2005). "Moderation of the Effect of Adolescent-Onset Cannabis Use on Adult Psychosis by a Functional Polymorphism in the Catechol-O-Methyltransferase Gene: Longitudinal Evidence of a Gene X Environment Interaction". Biological Psychiatry 57 (10): 1117–27. doi:10.1016/j.biopsych.2005.01.026. PMID 15866551.
- ^ Zammit, S.; Spurlock, G.; Williams, H.; Norton, N.; Williams, N.; O'Donovan, M. C.; Owen, M. J. (2007). "Genotype effects of CHRNA7, CNR1 and COMT in schizophrenia: interactions with tobacco and cannabis use". The British Journal of Psychiatry 191 (5): 402–7. doi:10.1192/bjp.bp.107.036129. PMID 17978319. Lay summary – MedWireNews.
- ^ Arseneault, L.; Cannon, M; Witton, J; Murray, RM (2004). "Causal association between cannabis and psychosis: examination of the evidence". The British Journal of Psychiatry 184 (2): 110–117. doi:10.1192/bjp.184.2.110. PMID 14754822.
- ^ Laqueille, X. (2009). "Le cannabis est-il un facteur de vulnérabilité des troubles schizophrènes ?". Archives de Pédiatrie 16 (9): 1302–5. doi:10.1016/j.arcped.2009.03.016.
- ^ Kawohl, W; Rössler, W (2008). "Cannabis and Schizophrenia: new findings in an old debate". Neuropsychiatrie : Klinik, Diagnostik, Therapie und Rehabilitation : Organ der Gesellschaft Osterreichischer Nervenarzte und Psychiater 22 (4): 223–9. PMID 19080993.
- ^ Degenhardt L, Hall W, Lynskey M (2001) (PDF). Comorbidity between cannabis use and psychosis: Modelling some possible relationships. Sydney: National Drug and Alcohol Research Centre. ISBN 07-334-1792-2. OCLC 50418990. http://ndarc.med.unsw.edu.au/NDARCWeb.nsf/resources/TR_18/$file/TR.121.PDF. Retrieved 2006-08-19.
- ^ Coulston, C; Perdices, M; Tennant, C (2007). "The neuropsychological correlates of cannabis use in schizophrenia: Lifetime abuse/dependence, frequency of use, and recency of use". Schizophrenia Research 96 (1–3): 169–184. doi:10.1016/j.schres.2007.08.006. PMID 17826035.
- ^ Jayanthi, S; Buie, S; Moore, S; Herning, R I; Better, W; Wilson, N M; Contoreggi, C; Cadet, J L (2008). "Heavy marijuana users show increased serum apolipoprotein C-III levels: evidence from proteomic analyses". Molecular Psychiatry 15 (1): 101–112. doi:10.1038/mp.2008.50. PMC 2797551. PMID 18475272. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2797551. Lay summary – Reuters (May 13, 2008).
- ^ Yucel, M.; Solowij, N.; Respondek, C.; Whittle, S.; Fornito, A.; Pantelis, C.; Lubman, D. I. (2008). "Regional Brain Abnormalities Associated With Long-term Heavy Cannabis Use". Archives of General Psychiatry 65 (6): 694–701. doi:10.1001/archpsyc.65.6.694. PMID 18519827.
- ^ Chang, L. (2006). "Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation". Brain 129 (5): 1096–1112. doi:10.1093/brain/awl064.
- ^ Ellgren, Maria (9 February 2007) (in English and Swedish). Neurobiological effects of early life cannabis exposure in relation to the gateway hypothesis. Stockholm. ISBN 978-91-7357-064-0. http://diss.kib.ki.se/2007/978-91-7357-064-0/.[page needed]
- ^ Ellgren, Maria; Spano, Sabrina M; Hurd, Yasmin L (2006). "Adolescent Cannabis Exposure Alters Opiate Intake and Opioid Limbic Neuronal Populations in Adult Rats". Neuropsychopharmacology 32 (3): 607–615. doi:10.1038/sj.npp.1301127. PMID 16823391.
- ^ Darke, Shane; Duflou, Johan; Torok, Michelle (2009). "Drugs and violent death: comparative toxicology of homicide and non-substance toxicity suicide victims". Addiction 104 (6): 1000–1005. doi:10.1111/j.1360-0443.2009.02565.x. PMID 19466923.
- ^ Price, C.; Hemmingsson, T.; Lewis, G.; Zammit, S.; Allebeck, P. (2009). "Cannabis and suicide: longitudinal study". The British Journal of Psychiatry 195 (6): 492–497. doi:10.1192/bjp.bp.109.065227. PMID 19949196.
- ^ Fellermeier, M; Zenk, MH (1998). "Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol". FEBS Letters 427 (2): 283–5. doi:10.1016/S0014-5793(98)00450-5. PMID 9607329.
- ^ Marks, M. D.; Tian, L.; Wenger, J. P.; Omburo, S. N.; Soto-Fuentes, W.; He, J.; Gang, D. R.; Weiblen, G. D. et al. (2009). "Identification of candidate genes affecting Δ9-tetrahydrocannabinol biosynthesis in Cannabis sativa". Journal of Experimental Botany 60 (13): 3715–26. doi:10.1093/jxb/erp210. PMC 2736886. PMID 19581347. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2736886.
- ^ Huestis, M. A. (2005). "Pharmacokinetics and Metabolism of the Plant Cannabinoids, Δ9-Tetrahydrocannibinol, Cannabidiol and Cannabinol". Cannabinoids. Handbook of Experimental Pharmacology 168 (168): 657–90. doi:10.1007/3-540-26573-2_23. ISBN 3-540-22565-X. PMID 16596792.
- ^ Schwilke, E. W.; Schwope, D. M.; Karschner, E. L.; Lowe, R. H.; Darwin, W. D.; Kelly, D. L.; Goodwin, R. S.; Gorelick, D. A. et al. (2009). "Δ9-Tetrahydrocannabinol (THC), 11-Hydroxy-THC, and 11-Nor-9-carboxy-THC Plasma Pharmacokinetics during and after Continuous High-Dose Oral THC". Clinical Chemistry 55 (12): 2180–2189. doi:10.1373/clinchem.2008.122119. PMC 3196989. PMID 19833841. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3196989.
- ^ Röhrich, J; Schimmel, I; Zörntlein, S; Becker, J; Drobnik, S; Kaufmann, T; Kuntz, V; Urban, R (2010). "Concentrations of Δ9-Tetrahydrocannabinol and 11-Nor-9-Carboxytetrahydrocannabinol in Blood and Urine After Passive Exposure to Cannabis Smoke in a Coffee Shop". Journal of Analytical Toxicology 34 (4): 196–203. PMID 20465865.
- ^ Baselt, R. (2011). Disposition of Toxic Drugs and Chemicals in Man (9th ed.). Seal Beach, CA: Biomedical Publications. pp. 1644–8.
- ^ "Marinol - the Legal Medical Use for the Marijuana Plant". Drug Enforcement Administration. http://www.usdoj.gov/dea/ongoing/marinol.html. Retrieved 2011-04-20.
- ^ Eustice, Carol (1997-08-12). "Medicinal Marijuana: A Continuing Controversy". About.com. http://arthritis.about.com/cs/medmarijuana/a/marijuanadebate.htm. Retrieved 2011-04-20.
- ^ Pickens, JT (1981). "Sedative activity of cannabis in relation to its delta'-trans-tetrahydrocannabinol and cannabidiol content". British journal of pharmacology 72 (4): 649–56. PMC 2071638. PMID 6269680. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2071638.
- ^ Burns, T. L; Ineck, JR (2006). "Cannabinoid Analgesia as a Potential New Therapeutic Option in the Treatment of Chronic Pain". Annals of Pharmacotherapy 40 (2): 251–260. doi:10.1345/aph.1G217. PMID 16449552.
- ^ MARINOL (dronabinol) capsule drug label/data at Daily Med from U.S. National Library of Medicine, National Institutes of Health.
- ^ McKim, William A (2002). Drugs and Behavior: An Introduction to Behavioral Pharmacology (5th ed.). Prentice Hall. p. 400. ISBN 0-13-048118-1.
- ^ Greenberg, Gary (2005-11-01). "Respectable Reefer". Mother Jones. http://motherjones.com/politics/2005/11/respectable-reefer. Retrieved 8 April 2010.
- ^ Calhoun, SR; Galloway, GP; Smith, DE (1998). "Abuse potential of dronabinol (Marinol)". Journal of psychoactive drugs 30 (2): 187–96. doi:10.1080/02791072.1998.10399689. PMID 9692381.[non-primary source needed]
- ^ "Petition to Reschedule Cannabis (Marijuana)". Coalition for Rescheduling Cannabis. October 9, 2002. http://www.drugscience.org/PDF/Petition_Final_2002.pdf.[non-primary source needed]
- Further reading
- Calhoun SR, Galloway GP, Smith DE (1998). "Abuse potential of dronabinol (Marinol)". J Psychoactive Drugs 30 (2): 187–96. doi:10.1080/02791072.1998.10399689. PMID 9692381.
- DEA Moves Marinol To Schedule Three, But Leaves Marijuana in Schedule One. The Magic of Sesame Oil, Richard Cowan, MarijuanaNews.Com.
- Petition to Reschedule Cannabis (Marijuana) per 21 CFR §1308.44(b), Filed October 9, 2002 with the DEA by the Coalition for Rescheduling Cannabis.
External links
Cannabinoids Plant cannabinoids Cannabinoid metabolites 8,11-DiOH-THC · 11-COOH-THC · 11-OH-THC
Endogenous cannabinoids Arachidonoyl ethanolamide (Anandamide or AEA) · 2-Arachidonoylglycerol (2-AG) · 2-Arachidonyl glyceryl ether (noladin ether) · Virodhamine · Palmitoylethanolamide (PEA) · N-Arachidonoyl dopamine (NADA) · Oleamide · RVD-Hpα
Synthetic cannabinoid
receptor agonistsClassical cannabinoids
(Dibenzopyrans)A-40174 · A-41988 · A-42574 · Ajulemic acid · AM-087 · AM-411 · AM-855 · AM-905 · AM-906 · AM-919 · AM-926 · AM-938 · AM-4030 · AMG-1 · AMG-3 · AMG-36 · AMG-41 · Dexanabinol (HU-211) · DMHP · Dronabinol · HHC · HU-210 · JWH-051 · JWH-133 · JWH-139 · JWH-161 · JWH-229 · JWH-359 · KM-233 · L-759,633 · L-759,656 · Levonantradol (CP 50,5561) · Nabazenil · Nabidrox (Canbisol) · Nabilone · Nabitan · Naboctate · O-581 · O-774 · O-806 · O-823 · O-1057 · O-1125 · O-1238 · O-2365 · O-2372 · O-2373 · O-2383 · O-2426 · O-2484 · O-2545 · O-2694 · O-2715 · O-2716 · O-3223 · O-3226 · Parahexyl · Perrottetinene · Pirnabine · THC-O-acetate · THC-O-phosphate
Nonclassical cannabinoidsBenzoylindoles1-Butyl-3-(2-methoxybenzoyl)indole · 1-Butyl-3-(4-methoxybenzoyl)indole · 1-Pentyl-3-(2-methoxybenzoyl)indole · AM-630 · AM-679 · AM-694 · AM-1241 · AM-2233 · GW-405,833 (L-768,242) · Pravadoline · RCS-4 · WIN 54,461
NaphthoylindolesNaphthylmethylindolesJWH-175 · JWH-184 · JWH-185 · JWH-192 · JWH-194 · JWH-195 · JWH-196 · JWH-197 · JWH-199
PhenylacetylindolesCannabipiperidiethanone · JWH-167 · JWH-203 · JWH-249 · JWH-250 · JWH-251 · JWH-302 · RCS-8
NaphthoylpyrrolesEicosanoidsAM-883 · Arachidonyl-2'-chloroethylamide (ACEA) · Arachidonylcyclopropylamide (ACPA) · Methanandamide (AM-356) · O-585 · O-689 · O-1812 · O-1860 · O-1861
Others(1-Pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone · N-(S)-Fenchyl-1-(2-morpholinoethyl)-7-methoxyindole-3-carboxamide · A-796,260 · A-834,735 · A-836,339 · Abnormal cannabidiol · AB-001 · AM-1248 · AZ-11713908 · BAY 38-7271 · BAY 59-3074 · CB-13 · CB-86 · GW-842,166X · JWH-171 · JWH-176 · JTE 7-31 · Leelamine · MDA-19 · O-1918 · O-2220 · Org 28312 · Org 28611 · SER-601 · VSN-16 · WIN 56,098
Allosteric modulators of
cannabinoid receptorsOrg 27569 · Org 27759 · Org 29647
Endocannabinoid
activity enhancersAM-404 · CAY-10401 · CAY-10429 · JZL184 · JZL195 · Arachidonoyl serotonin · O-1624 · PF-04457845 · PF-622 · PF-750 · PF-3845 · PHOP · URB-447 · URB-597 · URB-602 · URB-754 · Genistein · Arvanil · Olvanil · Kaempferol · Biochanin A
Cannabinoid receptor
antagonists and
inverse agonistsAM-251 · AM-281 · AM-630 · BML-190 · CAY-10508 · CB-25 · CB-52 · CB-86 · Drinabant · Hemopressin · Ibipinabant (SLV319) · JTE-907 · LY-320,135 · Taranabant (MK-0364) · MK-9470 · NESS-0327 · O-1184 · O-1248 · O-2050 · O-2654 · Otenabant · Rimonabant (SR141716) · SR144528 · Surinabant (SR147778) · TM-38837 · VCHSR
Hallucinogens Psychedelics
5-HT2AR agonists- Lysergamides: AL-LAD
- ALD-52
- BU-LAD
- CYP-LAD
- DAM-57
- Diallyllysergamide
- Ergometrine (Ergonovine, Ergobasine)
- ETH-LAD
- LAE-32
- LSA (Ergine, Lysergamide)
- LSD
- LSH
- LPD-824
- LSM-775
- Lysergic Acid 2-Butyl Amide
- Lysergic Acid 2,4-Dimethylazetidide
- Lysergic Acid 3-Pentyl Amide
- Methylergometrine
- Methylisopropyllysergamide
- Methysergide
- MLD-41
- PARGY-LAD
- PRO-LAD;
- Phenethylamines: Aleph
- 2C-B
- 2C-B-Dragonfly
- 2C-B-FLY
- 2C-C-FLY
- 2C-D-FLY
- 2C-E-FLY
- 2C-I-FLY
- 2CBFly-NBOMe
- 2C-T-7-FLY
- 2C-C
- 2C-C-NBOMe
- 2C-CN-NBOMe
- 2C-D
- 2CD-5EtO
- 2C-D-NBOMe
- 2C-E
- 2C-EF
- 2C-E-NBOMe
- 2C-F
- 2C-F-NBOMe
- 2C-G
- 2C-G-NBOMe
- 2C-H-NBOMe
- 2C-I
- 2C-N
- 2C-N-NBOMe
- 2C-O
- 2C-O-4
- 2C-P
- 2C-T
- 2C-T-2
- 2C-T-4
- 2C-T-4-NBOMe
- 2C-T-7
- 2C-T-7-NBOH
- 2C-T-8
- 2C-T-9
- 2C-T-13
- 2C-T-15
- 2C-T-17
- 2C-T-21
- 2C-TFM
- 2C-TFM-NBOMe
- 2C-YN
- 2CBCB-NBOMe
- 25B-NBOMe
- 25I-NBMD
- 25I-NBOH
- 25I-NBOMe
- 3C-E
- 3C-P
- 5-APB
- 5-APDB
- 6-APB
- 6-APDB
- Br-DFLY
- DESOXY
- DMMDA
- DMMDA-2
- DOB
- DOB-FLY
- DOM-FLY
- DOC
- DOEF
- DOET
- DOF
- DOI
- DOM
- DON
- DOPR
- DOTFM
- Escaline
- Ganesha
- HOT-2
- HOT-7
- HOT-17
- IAP
- Isoproscaline
- Jimscaline
- Lophophine
- MDA
- MDEA
- MDMA
- MMA
- MMDA
- MMDA-2
- MMDA-3a
- MMDMA
- Macromerine
- Mescaline
- Methallylescaline
- NBOMe-mescaline
- Proscaline
- TCB-2
- TFMFly
- TMA;
- Piperazines: pFPP
- TMFPP;
- Tryptamines: 1-Methyl-5-methoxy-diisopropyltryptamine
- 2,N,N-TMT
- 4-HO-5-MeO-DMT
- 4-Acetoxy-DET
- 4-Acetoxy-DIPT
- 4-Acetoxy-DMT
- 4-Acetoxy-DPT
- 4-Acetoxy-MiPT
- 4-HO-DPT
- 4-HO-MET
- 4-Propionyloxy-DMT
- 4-HO-MPMI
- 5-Me-MIPT
- 5-N,N-TMT
- 5-AcO-DMT
- 5-MeO-2,N,N-TMT
- 5-MeO-α,N,N-TMT
- 5-MeO-α-ET
- 5-MeO-α-MT
- 5-MeO-DALT
- 5-MeO-DET
- 5-MeO-DIPT
- 5-MeO-DMT
- 5-MeO-DPT
- 5-MeO-EiPT
- 5-MeO-MET
- 5-MeO-MIPT
- 5-MeO-MPMI
- 7,N,N-TMT
- α,N,N-TMT
- α-ET
- α-MT
- AL-37350A
- Baeocystin
- Bufotenin
- DALT
- DBT
- DCPT
- DET
- DIPT
- DMT
- DPT
- EiPT
- Ethocin
- Ethocybin
- Iprocin
- MET
- Miprocin
- MIPT
- Norbaeocystin
- PiPT
- Psilocin
- Psilocybin;
- Others: AL-38022A
- Ibogaine
- Noribogaine
- Voacangine
Dissociatives
NMDAR antagonists- Arylcyclohexylamines: 3-MeO-PCP
- 4-MeO-PCP
- Dieticyclidine
- Esketamine
- Eticyclidine
- Gacyclidine
- Ketamine
- Methoxetamine
- Neramexane
- Phencyclidine
- PCPr
- Rolicyclidine
- Tenocyclidine
- Tiletamine;
- Morphinans: Dextrallorphan
- Dextromethorphan
- Dextrorphan
- Methorphan (Racemethorphan)
- Racemorphan;
- Others: 2-MDP
- 8A-PDHQ
- Aptiganel
- Dexoxadrol
- Dizocilpine (MK-801)
- Etoxadrol
- Ibogaine
- Midafotel
- NEFA
- Nitrous Oxide
- Noribogaine
- Perzinfotel
- Remacemide
- Selfotel
- Xenon
Deliriants
mAChR antagonists- 3-Quinuclidinyl benzilate
- Atropine
- Benactyzine
- Benzatropine
- Benzydamine
- Biperiden
- Brompheniramine
- CAR-226,086
- CAR-301,060
- CAR-302,196
- CAR-302,282
- CAR-302,368
- CAR-302,537
- CAR-302,668
- Chlorpheniramine
- Chloropyramine
- Clemastine
- CS-27349
- Cyclizine
- Cyproheptadine
- Dicyclomine (Dicycloverine)
- Dimenhydrinate
- Diphenhydramine
- Ditran
- Doxylamine
- EA-3167
- EA-3443
- EA-3580
- EA-3834
- Elemicin
- Flavoxate
- Hydroxyzine
- Hyoscyamine
- Meclizine
- Myristicin
- N-Ethyl-3-piperidyl benzilate
- N-Methyl-3-piperidyl benzilate
- Pyrilamine
- Orphenadrine
- Oxybutynin
- Pheniramine
- Phenyltoloxamine
- Procyclidine
- Promethazine
- Scopolamine (Hyoscine)
- Tolterodine
- Trihexyphenidyl
- Tripelennamine
- Triprolidine
- WIN-2299
Miscellaneous - Apomorphine
- Bromocriptine
- Cabergoline
- Lisuride
- Memantine
- Pergolide
- Piribedil
- Pramipexole
- Ropinirole
- Rotigotine
- Butane
- Chloroform
- Diethyl Ether (Ether)
- Enflurane
- Freon
- Gasoline (Petrol)
- Kerosene (Paraffin)
- Propane
- Toluene
κOR agonists- 2-EMSB
- 2-MMSB
- Alazocine
- Bremazocine
- Butorphanol
- Cyclazocine
- Cyprenorphine
- Dextrallorphan
- Dezocine
- Enadoline
- Herkinorin
- HZ-2
- Ibogaine
- Ketazocine
- Metazocine
- Nalbuphine
- Nalorphine
- Noribogaine
- Pentazocine
- Phenazocine
- Salvinorin A
- Spiradoline
- Tifluadom
- U-50,488
- U-69,593
- Dextrallorphan
- Dextromethorphan
- Dextrorphan
- Noscapine (Narcotine)
OthersAntiemetics (A04) 5-HT3 Antagonists Alosetron • Azasetron • Bemesetron • Cilansetron • Clozapine • Dazopride • Dolasetron • Granisetron • Lerisetron • Metoclopramide • Mianserin • Mirtazapine • Olanzapine • Ondansetron • Palonosetron • Ramosetron • Ricasetron • Tropisetron • ZatosetronCB1 Agonists (Cannabinoids) D2/D3 Antagonists H1 Antagonists (Antihistamines) mACh Antagonists (Anticholinergics) NK1 Antagonists Others Ancient anaesthesia Plants/animals Aconite • Castoreum • Cannabis • Coca • Deadly nightshade • Henbane • Lactucarium • Mandrake • Metel nut • Opium • Poison hemlock • Saussurea • Toloatzin • WillowPeople Abulcasis • Avenzoar • Avicenna • Celsus • Dioscorides • Galen • Hippocrates • Rhazes • Sabuncuoğlu • Sushrutha • Theophrastus • ZhangCompounds Aconitine • Δ9-THC • Atropine • Cocaine • Coniine • Hyoscyamine • Morphine • Salicylate • ScopolamineCategories:- Amorphous solids
- Antiemetics
- Entheogens
- Cannabinoids
- Euphoriants
- Phenols
- Benzochromenes
Wikimedia Foundation. 2010.