- Chloropyramine
-
Chloropyramine 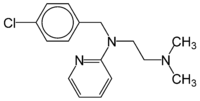
Systematic (IUPAC) name N-[(4-chlorophenyl)methyl]-N-[2-(dimethylamino)ethyl]pyridin-2-amine Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. C Legal status ? Identifiers CAS number 59-32-5 ATC code D04AA09 R06AC03 PubChem CID 25295 DrugBank DB08800 ChemSpider 23628 
UNII 2K3L8O9SOV 
KEGG D07195 
Chemical data Formula C16H20ClN3 Mol. mass 289.803 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Chloropyramine is a classical ("old" or first generation) antihistamine drug approved in some Eastern European countries for the treatment of allergic conjunctivitis, allergic rhinitis, bronchial asthma, and other atopic (allergic) conditions. Related indications for clinical use include Quincke's edema, allergic reactions to insect bites, food and drug allergies, and anaphylactic shock.
Chloropyramine is known as a competitive reversible H1-receptor antagonist (also known as an H1 inverse agonist), meaning that it exerts its pharmacological action by competing with histamine for the H1 subtype histamine receptor. By blocking the effects of histamine, the drug inhibits the vasodilation, increased vascular permeability, and tissue edema associated with histamine release in the tissue. The H1-antagonistic properties of chloropyramine can be used by researchers for the purposes of blocking the effects of histamine on cells and tissues. In addition, chloropyramine has some anticholinergic properties.
Chloropyramine's anticholinergic properties and the fact that it can pass through the blood-brain barrier are linked to its clinical side effects: drowsiness, weakness, vertigo, fatigue, dryness in the mouth, constipation, and rarely - visual disturbances and increase of intraocular pressure.
Contents
Clinical dosage and administration
In cases of severe allergic reactions, chloropyramine can be injected intramuscularly or intravenously. Oral administration: In adults, 25 mg can be taken 3 to 4 times daily (up to 150 mg); in children over 5 years-old, 25 mg can be taken 2 to 3 times daily. For external application, the skin or the conjuctiva of the eye can be treated up to several times a day by applying a thin layer of cream or ointment containing 1% chloropyramine hydrochloride.
Contraindications
Contraindications for parenteral or oral administration include adenoma of the prostate, acute peptic ulcer, pyloric and duodenal stenosis, glaucoma, pregnancy, breast-feeding.
Special warnings and precautions
Chloropyramine should not be used internally with alcohol, sedative drugs and hypnotics because of the potentiation of the effects. It should be used with caution in patients suffering from hyperthyroidism, cardiovascular diseases, and bronchial asthma. In children, it can induce agitation, and in many adult patients dizziness may be observed. Because of the pronounced sedative effect the preparation should be prescribed cautiously in drivers and people working with machines.
Drug interactions
Chloropyramine should not be used internally with MAO inhibitors. Because of its anticholinergic activity, concurrent administration with cholinomimetics is not advisable. General anesthetics, analgesic agents and psycholeptics potentiate the sedative effect of chloropyramine.
Chemistry
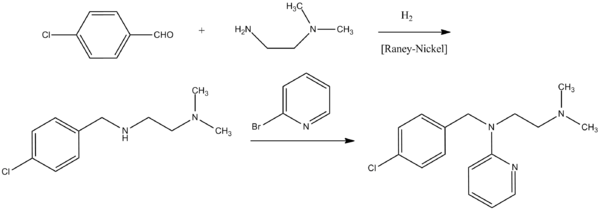
Chloropyramine, N-(4-chlorobenzyl)-N′,N′-dimethyl-N-2-pyridylethylenediamine, is synthesized by reacting 2-bromopyridine with N-(4-chlorobenzyl)-N′,N′-dimethylethylenediamine. N-(4-Chlorobenzyl)-N′,N′-dimethylethylenediamine, in turn, is synthesized by condensation of 4-chlorobenzaldehyde with N,N-dimethylethylenediamine followed by the subsequent reduction of the imine group.
- H.L. Kenneth, U.S. Patent 2,569,314 (1951).
- E.M. Cates, R.F. Phillips, U.S. Patent 2,607,778 (1952).
- J.R. Geigy AG., CH 264754 (1950).
- J.R. Geigy AG., CH 266234 (1950).
- J. R. Geigy AG., CH 266235 (1950).
- J.R. Geigy AG., GB 651596 (1951).
- Vaughan, J. R.; Anderson, G. W.; Clapp, R. C.; Clark, J. H.; English, J. P.; Howard, K. L.; Marson, H. W.; Sutherland, L. H. et al. (1949). "Antihistamine agents; halogenated N,N-dimethyl-N-benzyl-N-(2-pyridyl)-ethylenediamines". Journal of Organic Chemistry 14 (2): 228. doi:10.1021/jo01154a006. PMID 18117722.
References
Antipruritics (D04) Antihistamines for topical use Thonzylamine - Mepyramine - Thenalidine - Tripelennamine - Chloropyramine - Promethazine - Tolpropamine - Dimetindene - Clemastine - Bamipine - Isothipendyl - Diphenhydramine - ChlorphenoxamineAnesthetics for topical use Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- H1 receptor antagonists
- Organochlorides
- Amines
- Pyridines
Wikimedia Foundation. 2010.
Look at other dictionaries:
chloropyramine — noun A particular first generation antihistamine drug … Wiktionary
chloropyramine — An H1 antihistaminic agent … Medical dictionary
H1 antagonist — An H1 antagonist is a histamine antagonist of the H1 receptor that serves to reduce or eliminate effects mediated by histamine, an endogenous chemical mediator released during allergic reactions. Agents where the main therapeutic effect is… … Wikipedia
Diphenhydramine — Systematic (IUPAC) name 2 (diphenylmethoxy) N,N dimethylethanamine Clinical data Trade names Benadryl … Wikipedia
Hydroxyzine — Systematic (IUPAC) name (±) 2 (2 {4 [(4 chlorophenyl) phe … Wikipedia
Cetirizine — Systematic (IUPAC) name (±) [2 [4 [(4 chlorophenyl)phenylmethyl] 1 piperazinyl]ethoxy]acetic acid Clinical data Trade names … Wikipedia
Cyclizine — Systematic (IUPAC) name 1 benzhydryl 4 methyl piperazine … Wikipedia
Azelastine — Systematic (IUPAC) name (RS) 4 [(4 chlorophenyl)methyl] 2 (1 methylazepan 4 yl) phthalazin 1 one Clinical data Trade names … Wikipedia
Diphenylpyraline — Systematic (IUPAC) name 4 benzhydryloxy 1 methyl piperidine Clinical data AHFS/Drugs.com … Wikipedia
Oxatomide — Systematic (IUPAC) name 1 {3 [4 (diphenylmethyl)piperazin 1 yl]propyl} 1,3 dihydro 2H benzimidazol 2 one Clinical data AHFS/Drugs.com … Wikipedia