- Betahistine
-
Betahistine 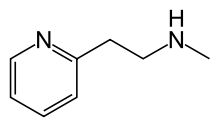
Systematic (IUPAC) name N-methyl-2-(pyridin-2-yl)ethanamine Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status ? Routes Oral Pharmacokinetic data Protein binding Very low Metabolism To 2-(2-aminoethyl)pyridine and 2-pyridylacetic acid[1] Half-life 3–4 hours Excretion Renal Identifiers CAS number 5638-76-6 ATC code N07CA01 PubChem CID 2366 DrugBank DB06698 ChemSpider 2276 
UNII X32KK4201D 
KEGG D07522 
ChEBI CHEBI:35677 
ChEMBL CHEMBL24441 
Chemical data Formula C8H12N2 Mol. mass 136.194 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Betahistine hydrochloride (brand names Serc, Hiserk, Betaserc) is an antivertigo drug. It was first registered in Europe in 1970 for the treatment of Ménière's disease. It is commonly prescribed to patients with balance disorders or to alleviate vertigo symptoms associated with Ménière's disease.
Contents
Chemistry
Betahistine chemically is 2-[2-(methylamino)ethyl]pyridine, and is formulated as the dihydrochloride salt. Its structure closely resembles that of phenethylamine and histamine.
Pharmacokinetics
Betahistine comes in tablet form and is taken orally. It is rapidly and completely absorbed. The mean plasma half-life is 3–4 hours, and excretion is virtually complete in the urine within 24 hours. Plasma protein binding is very low. Betahistine is transformed into aminoethylpyridine and hydroxyethylpyridine and excreted with the urine as pyridylacetic acid. There is some evidence that one of these metabolites, aminoethylpyridine, may be active and exert effects similar to those of betahistine on ampullar receptors.[2]
Mode of action
Betahistine has a very strong affinity as an antagonist for histamine H3 receptors and a weak affinity as an agonist for histamine H1 receptors. Betahistine seems to dilate the blood vessels within the middle ear which can relieve pressure from excess fluid and act on the smooth muscle.
Betahistine has two modes of action. Primarily, it has a direct stimulating (agonistic) effect on H1 receptors located on blood vessels in the inner ear. This gives rise to local vasodilation and increased permeability, which helps to reverse the underlying problem of endolymphatic hydrops.
In addition, betahistine has a powerful antagonistic effects at H3 receptors, and increases the levels of neurotransmitters released from the nerve endings. This is thought to have two consequences;
- The increased amounts of histamine released from histaminergic nerve endings can stimulate H1 receptors, thus augmenting the direct agonistic effects of betahistine on these receptors. This explains the potent vasodilatory effects of betahistine in the inner ear, which are well documented.
- It is postulated that betahistine increases the levels of neurotransmitters such as serotonin in the brainstem, which inhibits the activity of vestibular nuclei.
Side effects
- Headache.
- Low level of gastric side effects.
- Nausea can be a side effect, but the patient is generally already experiencing nausea due to the vertigo so it goes largely unnoticed.
- Decreased appetite, leading to weight loss
- Patients taking betahistine hydrochloride may experience several hypersensitivity and allergic reactions. In the November 2006 issue of "Drug Safety," Dr. Sabine Jeck-Thole and Dr. Wolfgang Wagner reported that betahistine hydrochloride may cause several allergic and skin-related side effects. These include rash in several areas of the body; itching and hives; and swelling of the face, tongue and mouth. Other hypersensitivity reactions reported include tingling, numbness, burning sensations, shortness of breath and labored breathing. The study authors suggest that hypersensitivity reactions may be a direct result of betahistine's role in increasing histamine levels throughout the body. Hypersensitivity reactions quickly subside after betahistine has been discontinued.
Digestive Side Effects Betahistine may also cause several digestive-related side effects. The package insert for Serc, a trade name for betahistine, states that patients may experience several gastrointestinal side effects. These may include nausea, upset stomach, vomiting, diarrhea and stomach cramping. These symptoms are usually not serious and subside in between doses. Patients experiencing chronic digestive problems may lower their dose to the minimum effective range and by taking betahistine with meals. Additional digestive problems may require that patients consult their physician in order to possibly find a suitable alternative. Other Side Effects Patients taking betahistine may experience several other side effects ranging from mild to serious. The package insert for Serc states that patients may experience nervous system side effects, including convulsions, daytime sleepiness, confusion and hallucinations. Some nervous system events may also partly be attributable to the underlying condition rather than the medication used to treat it. Other side effects listed in the package insert include low blood pressure and heart rhythm irregularities. The study by Jeck-Thole and Wagner also reports that patients may experience headache and liver problems, including increased liver enzymes and bile flow disturbances. Any side effects that persist or outweigh the relief of symptoms of the original condition may warrant that the patient consult their physician to adjust or change the medication.
Read more: http://www.livestrong.com/article/270761-betahistine-hydrochloride-side-effects/#ixzz1QmvDG2WD http://www.livestrong.com/article/270761-betahistine-hydrochloride-side-effects/Contraindications
Betahistine is contraindicated for people with peptic ulcers or tumours of the adrenal gland, such as pheochromocytoma. People with bronchial asthma should be closely monitored.
Experimental uses
A new use for betahistine may be in the field of obesity management. Dr Nir Barak of the Rabin Medical Centre in Tel Aviv has undertaken trials[3] and it is reported (Telegraph, UK, 19 February 2007) that volunteers lost more than 1.5 kg/week over twelve weeks and experienced a distaste for fatty foods.
A recent Phase II clinical trial of the new drug in the U.S. suggests that women under the age of 50 who took Histalean (Betahistine) for 12 weeks lost 7 times the weight of those taking a placebo. What's most important to the researchers involved is that none of the 281 patients, males and females aged 18–65, complained of any serious side effects. The recent results were based on a double-blind, placebo-controlled study on people with a Body Mass Index ranging from 30 to 40. (A BMI of 30 and above indicate obesity.) The study was conducted at 19 investigation sites across the U.S. over a 12 week treatment period. The subgroup of high-dose Histalean (Betahistine)-treated women lost an average of 2.91% of their weight versus placebo group which lost only 0.4 %.[4]
Betahistine is being studied for the treatment of atypical depression at dosages from 50–300 mg/day due to its ability to antagonise H3 receptors and thus increase the release of serotonin and dopamine from nerve endings into the synaptic cleft.[5]
References
- ^ (French) "Betahistine Dichlorhydrate". BIAM. June 30, 1999. http://www.biam2.org/www/Sub1291.html. Retrieved on November 7, 2008.
- ^ Botta L, Mira E, Valli S, Zucca G, Perin P, Benvenuti C, Fossati A, Valli P (June 2001). "Effects of betahistine and of its metabolites on vestibular sensory organs.". Acta Otorhinolaryngol Ital. 21 (3 Suppl 66): 24–30. PMID 11677836.
- ^ Halle, Martyn (20 February 2007). "Vertigo pill is new obesity wonderdrug". London. http://www.dailymail.co.uk/pages/live/articles/health/healthmain.html?in_article_id=437291&in_page_id=1774. Retrieved 2007-06-20.
- ^ "New drug makes weight loss safer". http://www.brightsurf.com/news/headlines/33188/New_drug_makes_weight_loss_safer.html. Retrieved 2007-09-27.
- ^ http://clinicaltrials.gov/ct2/show/NCT00585585
Antivertigo preparations (N07C) Categories:- Vasodilators
- Pyridines
Wikimedia Foundation. 2010.
