- Meclozine
-
Meclozine 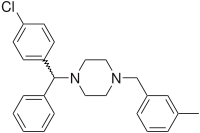
Systematic (IUPAC) name (R/S)-1-[(4-chlorophenyl)(phenyl)methyl]-4-(3-methylbenzyl)piperazine Clinical data MedlinePlus a682548 Pregnancy cat. B(US) Legal status OTC (US); OTC (Canada) Routes Oral, Sublingual/Buccal Pharmacokinetic data Metabolism hepatic Half-life 6 hours Identifiers CAS number 569-65-3 ATC code A04AB04 R06AE05 PubChem CID 4034 DrugBank APRD00354 ChemSpider 3894 
UNII 3L5TQ84570 
ChEMBL CHEMBL1623 Chemical data Formula C25H27ClN2 Mol. mass 390.948 g/mol SMILES eMolecules & PubChem Physical data Boiling point 230 °C (446 °F)  (what is this?) (verify)
(what is this?) (verify)Meclozine (INN,[1] or meclizine) is an antihistamine considered to be an antiemetic. It is sold under the brand names Bonine, Bonamine, Antivert, Postafen, Sea Legs, and Dramamine (Less Drowsy Formulation). Emesafene is a combination of meclozine (1/3) and pyridoxine (2/3). In Canada, Antivert Tab (which is no longer available) was a combination of meclozine and nicotinic acid.[2]
Contents
Classification
Meclozine is a first-generation antihistamine of the piperazine class. Meclozine is structurally and pharmacologically similar to buclizine, cyclizine, and hydroxyzine, but has a shorter half-life of 6 hours compared to cyclizine and hydroxyzine with about 20 hours. It is used as an antivertigo/antiemetic agent, specifically in the prevention and treatment of nausea, vomiting, and dizziness associated with motion sickness.[3]
Mechanism of action
Meclozine is an antagonist at H1 receptors. It possesses anticholinergic, central nervous system depressant, and local anesthetic effects. Its antiemetic and antivertigo effects are not fully understood, but its central anticholinergic properties are partially responsible. The drug depresses labyrinth excitability and vestibular stimulation, and it may affect the medullary chemoreceptor trigger zone.[3]
Uses
Meclozine is approved by the U.S. Food and Drug Administration (FDA) to treat symptoms of motion sickness and for management of vertigo that stems from diseases affecting the vestibular system. Meclozine's safety and efficacy in children younger than 12 years old has not been established, therefore use in this population is not recommended. Also, meclozine should be taken with caution in the elderly (older than 65 years) because of increased risk of confusion and amnesia.[4]
Sickness
Meclozine is effective in inhibiting the symptoms of motion sickness, such as nausea, vomiting, and dizziness. The recommended dose is 25–50 mg orally, taken 1 hour before travel. The dose may be repeated every 24 hours as needed.[3]
The drug is also safe for treating nausea in pregnancy[5] and is a first-line therapy for this use.[6][7] Doxylamine is similarly safe.
Vertigo
Meclozine may be effective in relieving vertigo experienced as a result of inner ear infections or other conditions. The recommended dose is 25–100 mg per day orally, separated into divided doses.[3]
Side effects
Some common side effects such as drowsiness, dry mouth, and tiredness may occur. Meclozine has been shown to have fewer dry mouth side effects than the traditional treatment for motion sickness, transdermal scopolamine.[8] A very serious allergic reaction to this drug is unlikely, but seek immediate medical attention if it occurs. Symptoms of a serious allergic reaction may include: rash, itching/swelling, severe dizziness, and/or trouble breathing.[9]
Drowsiness
Drowsiness may result as a side effect of taking meclozine. Users are advised not to operate heavy machinery while under the influence. The consumption of alcohol while under the influence of meclozine may result in additional drowsiness.
Special considerations in the elderly
As with any anticholinergic agent, meclozine may cause confusion or aggravate symptoms in those with dementia in the geriatric population (older than 65 years). Therefore caution should be used when administering meclozine to the elderly.[10]
Synthesis
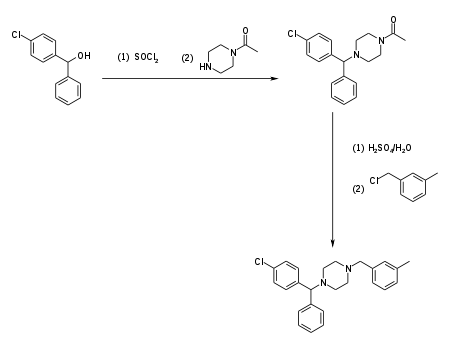
(4-Chlorphenyl)-phenylmethanol is halogenated with thionyl chloride before adding acetylpiperazine. The acetyl group is cleaved with diluted sulfuric acid. An N-alkylation of the piperzine ring with 3-methylbenzylchloride completes the synthesis.[11]
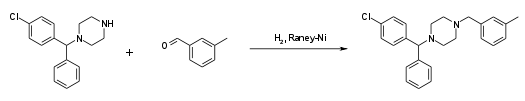
Alternatively, the last step can be replaced by a reductive N-alkylation with 3-methylbenzaldehyde. The reductive agent is hydrogen, and Raney nickel is used as a catalyst.[12][13]
Meclozine is obtained and used as a racemate, a 1:1 mixture of the two stereoisomers. Drug forms contain the dihydrochloride.
References
- ^ KEGG Drug: D08163
- ^ DrugBank. Drugbank: Drug card for Meclizine David Wishard: University of Alberta, Canada. Accessed November 7, 2010.
- ^ a b c d Clinical Pharmacology. Clinical Pharmacology, revised November 20, 2009, accessed November 7, 2010.[broken citation]
- ^ MICROMEDEX 2.0. Accessed November 7, 2010.[broken citation]
- ^ Källén B, Mottet I (2003). "Delivery outcome after the use of meclozine in early pregnancy". European Journal of Epidemiology 18 (7): 665–669. PMID 12952140. http://www.kluweronline.com/art.pdf?issn=0393-2990&volume=18&page=665. Retrieved 2010-09-17.
- ^ "Antiemetische Therapie bei Schwangerschaftserbrechen [Antiemetic therapy in pregnancy]" (in German). Arznei-Telegramm 40: 87–89. 2009. http://www.arznei-telegramm.de/html/2009_10/0910087_01.html.
- ^ Embryotox: Meclozin (German)
- ^ Dahl E, Offer-Ohlsen D, Lillevold PE, Sandvik L. Transdermal scopolamine, oral meclozine, and placebo in motion sickness. Clinical Pharmacology And Therapeutics [Clin Pharmacol Ther] 1984 Jul; Vol. 36 (1), pp. 116-20. Available from: MEDLINE: Ipswich, MA. PMID 6734040
- ^ Meclizine - oral, Antivert, D-vert, Dramamine II. Accessed November 7, 2010.
- ^ Merck Manuals, Online Medical Library: Meclizine (Drug Information Provided by Lexi-Comp), revised January 2010, accessed November 7, 2010.
- ^ J.-H. Fuhrkop, G. Li (2003). Organic Synthesis. Concepts and Methods. Wiley. p. 237. ISBN 978-3-527-30272-7.
- ^ US 2 709 169 (UCB, 1955)
- ^ A. Kleemann, J. Engel, B. Kutscher, D. Reichert (2001). Pharmaceutical Substances. Synthesis, Patents, Applications (4 ed.). Thieme. ISBN 3-13-115134-X.
External links
- University of Berlin's Institute of Biochemistry
- Numark Pharmacies Information Page on Meclozine
- Rxlist Medical Dictionary
Antiemetics (A04) 5-HT3 Antagonists Alosetron • Azasetron • Bemesetron • Cilansetron • Clozapine • Dazopride • Dolasetron • Granisetron • Lerisetron • Metoclopramide • Mianserin • Mirtazapine • Olanzapine • Ondansetron • Palonosetron • Ramosetron • Ricasetron • Tropisetron • ZatosetronCB1 Agonists (Cannabinoids) D2/D3 Antagonists H1 Antagonists (Antihistamines) mACh Antagonists (Anticholinergics) NK1 Antagonists Others Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximePiperazines Simple piperazines
(no additional rings)1-Cyclohexylpiperazine • Aminoethylpiperazine • Diethylcarbamazine • HEPPS • Midafotel • Piperazine • PIPESPhenylpiperazines Acaprazine • Antrafenine • Aripiprazole • Batoprazine • Bifeprunox • BRL-15,572 • Ciprofloxacin • CSP-2503 • Dapiprazole • DCPP • DMPP • Diphenylpiperazine • Dropropizine • EGIS-12,233 • Elopiprazole • Eltoprazine • Enpiprazole • Ensaculin • Etoperidone • Flesinoxan • Flibanserin • Fluprazine • Itraconazole • Ketoconazole • Levodropropizine • Lorpiprazole • mCPP • Mefway • MeOPP • Mepiprazole • Naftopidil • Naphthylpiperazine • Nefazodone • Niaprazine • Oxypertine • Pardoprunox • pCPP • pFPP • Posaconazole • PRX-00023 • S-14,506 • S-14,671 • S-15,535 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • Sonepiprazole • TFMPP • Tolpiprazole • Trazodone • Urapidil • Vesnarinone • Vilazodone • WAY-100,135 • WAY-100,635Benzylpiperazines 2C-B-BZP • Befuraline • Bifeprunox • Buclizine • BZP • Chlorbenzoxamine • DBZP • Fipexide • Imatinib • MBZP • MDBZP • Meclozine • Piberaline • Piribedil • Trimetazidine • VesnarinoneDiphenylalkylpiperazines
(benzhydrylalkylpiperazines)Almitrine • Amperozide • BRL-15,572 • Buclizine • BW373U86 • Cetirizine • Chlorbenzoxamine • Chlorcyclizine • Cinnarizine • Clocinizine • Cyclizine • DBL-583 • Diphenylmethylpiperazine • Dotarizine • DPI-221 • DPI-287 • DPI-3290 • GBR-12,783 • GBR-12,935 • GBR-13,069 • GBR-13,098 • GBR-13,119 • Hydroxyzine • Lidoflazine • Manidipine • Meclozine • Oxatomide • SNC-80 • VanoxerinePyrimidinylpiperazines Buspirone • Dasatinib • Eptapirone • Gepirone • Ipsapirone • Piribedil • Pyrimidinylpiperazine • Revospirone • Tandospirone • Tirilazad • Trimazosin • Umespirone • ZalospironePyridinylpiperazines Atevirdine • Azaperone • PyridinylpiperazineBenzo(iso)thiazolylpiperazines Tricyclics
(piperazine attached via side chain)Others 6-Nitroquipazine • Azimilide • Cinepazet • Cyclohexylpiperazine • Hexocyclium • Indinavir • JNJ-7777120 • Lodenafil • Mirodenafil • PB-28 • Quipazine • Ranolazine • SA-4503 • Sildenafil • Tadalafil • Vardenafil • VUF-6002 • ZipeprolCategories:- Antiemetics
- Piperazines
- Organochlorides
Wikimedia Foundation. 2010.
Look at other dictionaries:
Méclozine — Général Nom IUPAC (R/S) 1 [(4 chlorophényl)(phényl)méthyl] 4 (3 méthylbenzyl)pipérazine No CAS … Wikipédia en Français
meclozine — noun meclizine … Wiktionary
meclozine — n. an antihistamine drug used mainly to prevent and treat nausea and vomiting, particularly in motion sickness, and also to relieve allergic reactions. It is administered by mouth … Medical dictionary
meclozine — n. an antihistamine drug used to prevent and treat nausea and vomiting, particularly in motion sickness. It is administered by mouth … The new mediacal dictionary
meclozine — … Useful english dictionary
meclozine hydrochloride — SYN: meclizine hydrochloride … Medical dictionary
Меклозин (Meclozine) — антигистаминный препарат; используется в основном для предотвращения и лечения тошноты и рвоты, особенно при укачивании, а также для уменьшения проявлений аллергических реакций. Назначается внутрь. Источник: Медицинский словарь … Медицинские термины
МЕКЛОЗИН — (meclozine) антигистаминный препарат; используется в основном для предотвращения и лечения тошноты и рвоты, особенно при укачивании, а также для уменьшения проявлений аллергических реакций. Назначается внутрь … Толковый словарь по медицине
Chlorcyclizine — Systematic (IUPAC) name 1 [(4 chlorophenyl)(phenyl)methyl] 4 methylpiperazine Clinical data AHFS/Drugs.com … Wikipedia
Hydroxyzine — Systematic (IUPAC) name (±) 2 (2 {4 [(4 chlorophenyl) phe … Wikipedia