- Dasatinib
-
Dasatinib 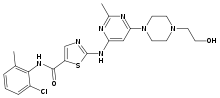
Systematic (IUPAC) name N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-
1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazole
carboxamide monohydrateClinical data AHFS/Drugs.com monograph MedlinePlus a607063 Licence data EMA:Link, US FDA:link Pregnancy cat. D(AU) D(US) Legal status ℞-only (US) Routes Oral Pharmacokinetic data Protein binding 96% Metabolism Hepatic Half-life 1.3 to 5 hours Excretion Fecal (85%), renal (4%) Identifiers CAS number 302962-49-8 
ATC code L01XE06 PubChem CID 3062316 DrugBank DB01254 ChemSpider 2323020 
UNII X78UG0A0RN 
KEGG D03658 
ChEBI CHEBI:49375 
ChEMBL CHEMBL1421 
Chemical data Formula C22H26ClN7O2S Mol. mass 488.01 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Dasatinib, previously known as BMS-354825, is a cancer drug produced by Bristol-Myers Squibb and sold under the trade name Sprycel. Dasatinib is an oral multi- BCR/ABL and Src family tyrosine kinase inhibitor approved for use in patients with chronic myelogenous leukemia (CML) after imatinib treatment and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). It is being evaluated for use in numerous other cancers, including advanced prostate cancer.
The drug is named after one of the inventor chemists, Jagabandhu Das, who was a member of the large discovery and development team at Bristol Myers Squibb.[1]
Contents
Origin and development
Efficacy
In a Phase I dose escalation study published in June 2006, dasatinib was tested in patients who were resistant to or who could not tolerate imatinib.[2] Complete hematological responses[3] were seen in 37 of 40 patients with chronic-phase CML. Major hematologic responses[4] were seen in 31 of 44 patients with accelerated-phase CML, CML in blast crisis, or Ph+ ALL.
Molecular targets
The main targets of dasatinib, are BCR/ABL, Src, c-Kit, ephrin receptors, and several other tyrosine kinases, but not erbB kinases such as EGFR or Her2.
Duration of benefit
Responses were maintained in 95% of patients with chronic-phase CML, with a median follow-up time of >12 months. In patients with accelerated-phase CML, 82% remained in remission, although with a median follow-up of only 5 months. Nearly all patients with CML in blast crisis or Ph+ ALL relapsed within 6 months.
Susceptible genotypes
Responses were seen in patients with all BCR/ABL genotypes, with the exception of T315I mutation, which confers resistance to both dasatinib, nilotinib and imatinib in vitro.
Toxicities
Neutropenia and myelosuppression were common toxic effects. Fifteen patients (of 84, ie 18%) in the above-mentioned study developed pleural effusions, which were felt to be a side effect of dasatinib. Some of these patients required thoracentesis or pleurodesis to treat the effusions. Other adverse events included mild to moderate diarrhea, peripheral edema, and headache. A small number of patients developed abnormal liver function tests which returned to normal without dose adjustments. Mild hypocalcemia was also noted, but did not appear to cause any significant problems.Several cases of pulmonary arterial hypertension (PAH) were found in patients treated with dasatinib.[6]
Adverse effects
On October 11, 2011 the U.S. Food and Drug Administration (FDA) announced that dasatinib may increase the risk of a rare but serious condition in which there is abnormally high blood pressure in the arteries of the lungs (pulmonary hypertension, PAH). Symptoms of PAH may include shortness of breath, fatigue, and swelling of the body (such as the ankles and legs). In reported cases, patients developed PAH after starting dasatinib, including after more than one year of treatment.
And information about this risk has been added to the Warnings and Precautions section of the Sprycel drug label.[7]
See also
- Discovery and development of Bcr-Abl tyrosine kinase inhibitors
References
- ^ Das J et al. (2006). "2-aminothiazole as a novel kinase inhibitor template. Structure-activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1- piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide (dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor". J Med Chem 49 (23): 6819–32. doi:10.1021/jm060727j. PMID 17154512.
- ^ Talpaz M, Shah NP, Kantarjian H, et al. (June 2006). "Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias". N. Engl. J. Med. 354 (24): 2531–41. doi:10.1056/NEJMoa055229. PMID 16775234. http://content.nejm.org/cgi/pmidlookup?view=short&pmid=16775234&promo=ONFLNS19.
- ^ Complete hematologic response was defined as normal white blood cell and platelet counts, no blasts in the peripheral blood, <5% myelocytes plus metamyelocytes in the peripheral blood, <20% basophils in the peripheral blood, and no extramedullary disease.
- ^ The definition of a major hematologic response was sufficiently abstruse that the reader is referred to the original article (Talpaz et al., 2006) for details.
- ^ Tokarski, J. S.; Newitt, J. A.; Chang, C. Y.; Cheng, J. D.; Wittekind, M.; Kiefer, S. E.; Kish, K.; Lee, F. Y. et al. (2006). "The Structure of Dasatinib (BMS-354825) Bound to Activated ABL Kinase Domain Elucidates Its Inhibitory Activity against Imatinib-Resistant ABL Mutants". Cancer Research 66 (11): 5790–5797. doi:10.1158/0008-5472.CAN-05-4187. PMID 16740718.
- ^ NHS - Healthcare News
- ^ FDA: Sprycel (dasatinib): Drug Safety Communication - Risk of Pulmonary Arterial Hypertension, 10/11/2011.
External links
- Summary Basis for Approval from the U.S. Food and Drug Administration Freedom of Information homepage
- Prescribing information from Bristol-Myers Squibb
- Sprycel Summary of Product Characteristics (from the European Medicines Agency website)
Targeted therapy / extracellular chemotherapeutic agents/antineoplastic agents (L01) CI monoclonal antibodies ("-mab") Others for solid tumorsTyrosine-kinase inhibitors ("-nib") ErbB: HER1/EGFR (Erlotinib, Gefitinib, Vandetanib) • HER1/EGFR and HER2/neu (Afatinib, Lapatinib, Neratinib)
RTK class III: C-kit and PDGFR (Axitinib, Pazopanib, Sunitinib, Sorafenib, Toceranib) • FLT3 (Lestaurtinib)
VEGFR (Axitinib, Cediranib, Pazopanib, Regorafenib, Semaxanib, Sorafenib, Sunitinib, Toceranib, Vandetanib)Other fusion protein against VEGF (Aflibercept) • proapoptotic peptide against ANXA2 and prohibitin (Adipotide) • exotoxin against IL-2 (Denileukin diftitox)M: NEO
tsoc, mrkr
tumr, epon, para
drug (L1i/1e/V03)
Piperazines Simple piperazines
(no additional rings)1-Cyclohexylpiperazine • Aminoethylpiperazine • Diethylcarbamazine • HEPPS • Midafotel • Piperazine • PIPESPhenylpiperazines Acaprazine • Antrafenine • Aripiprazole • Batoprazine • Bifeprunox • BRL-15,572 • Ciprofloxacin • CSP-2503 • Dapiprazole • DCPP • DMPP • Diphenylpiperazine • Dropropizine • EGIS-12,233 • Elopiprazole • Eltoprazine • Enpiprazole • Ensaculin • Etoperidone • Flesinoxan • Flibanserin • Fluprazine • Itraconazole • Ketoconazole • Levodropropizine • Lorpiprazole • mCPP • Mefway • MeOPP • Mepiprazole • Naftopidil • Naphthylpiperazine • Nefazodone • Niaprazine • Oxypertine • Pardoprunox • pCPP • pFPP • Posaconazole • PRX-00023 • S-14,506 • S-14,671 • S-15,535 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • Sonepiprazole • TFMPP • Tolpiprazole • Trazodone • Urapidil • Vesnarinone • Vilazodone • WAY-100,135 • WAY-100,635Benzylpiperazines 2C-B-BZP • Befuraline • Bifeprunox • Buclizine • BZP • Chlorbenzoxamine • DBZP • Fipexide • Imatinib • MBZP • MDBZP • Meclozine • Piberaline • Piribedil • Trimetazidine • VesnarinoneDiphenylalkylpiperazines
(benzhydrylalkylpiperazines)Almitrine • Amperozide • BRL-15,572 • Buclizine • BW373U86 • Cetirizine • Chlorbenzoxamine • Chlorcyclizine • Cinnarizine • Clocinizine • Cyclizine • DBL-583 • Diphenylmethylpiperazine • Dotarizine • DPI-221 • DPI-287 • DPI-3290 • GBR-12,783 • GBR-12,935 • GBR-13,069 • GBR-13,098 • GBR-13,119 • Hydroxyzine • Lidoflazine • Manidipine • Meclozine • Oxatomide • SNC-80 • VanoxerinePyrimidinylpiperazines Buspirone • Dasatinib • Eptapirone • Gepirone • Ipsapirone • Piribedil • Pyrimidinylpiperazine • Revospirone • Tandospirone • Tirilazad • Trimazosin • Umespirone • ZalospironePyridinylpiperazines Atevirdine • Azaperone • PyridinylpiperazineBenzo(iso)thiazolylpiperazines Tricyclics
(piperazine attached via side chain)Others 6-Nitroquipazine • Azimilide • Cinepazet • Cyclohexylpiperazine • Hexocyclium • Indinavir • JNJ-7777120 • Lodenafil • Mirodenafil • PB-28 • Quipazine • Ranolazine • SA-4503 • Sildenafil • Tadalafil • Vardenafil • VUF-6002 • ZipeprolCategories:- Orphan drugs
- Non-receptor tyrosine kinase inhibitors
- Thiazoles
- Piperazines
- Bristol-Myers Squibb
- Pyrimidines
- Organochlorides
- Anilides
- Amines
- Alcohols
Wikimedia Foundation. 2010.

