- Platelet-derived growth factor receptor
-
platelet-derived growth factor receptor, alpha polypeptide Identifiers Symbol PDGFRA Entrez 5156 HUGO 8803 OMIM 173490 RefSeq NM_006206 UniProt P16234 Other data Locus Chr. 4 q12 platelet-derived growth factor receptor, beta polypeptide 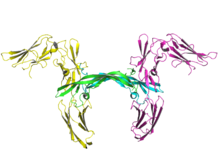
Ribbon image of two molecules of human PDGF receptor beta (yellow and magenta) in complex with dimeric PDGF-B (cyan and green).[1] Identifiers Symbol PDGFRB Alt. symbols PDGFR Entrez 5159 HUGO 8804 OMIM 173410 RefSeq NM_002609 UniProt P09619 Other data Locus Chr. 5 q31-q32 Platelet-derived growth factor receptors (PDGF-R) are cell surface tyrosine kinase receptors for members of the platelet-derived growth factor (PDGF) family. PDGF subunits -A and -B are important factors regulating cell proliferation, cellular differentiation, cell growth, development and many diseases including cancer.[2] There are two forms of the PDGF-R, alpha and beta each encoded by a different gene.[3] Depending on which growth factor is bound, PDGF-R homo- or heterodimerizes.[4]
Contents
Mechanism of action
The PDGF family consists of PDGF-A, -B, -C and -D, which form either homo- or heterodimers (PDGF-AA, -AB, -BB, -CC, -DD). The four PDGFs are inactive in their monomeric forms. The PDGFs bind to the protein tyrosine kinase receptors PDGF receptor-α and -β. These two receptor isoforms dimerize upon binding the PDGF dimer, leading to three possible receptor combinations, namely -αα, -ββ and -αβ. The extracellular region of the receptor consists of five immunoglobulin-like domains while the intracellular part is a tyrosine kinase domain. The ligand-binding sites of the receptors are located to the three first immunoglobulin-like domains. PDGF-CC specifically interacts with PDGFR-αα and -αβ, but not with -ββ, and thereby resembles PDGF-AB. PDGF-DD binds to PDGFR-ββ with high affinity, and to PDGFR-αβ to a markedly lower extent and is therefore regarded as PDGFR-ββ specific. PDGF-AA binds only to PDGFR-αα, while PDGF-BB is the only PDGF that can bind all three receptor combinations with high affinity.
Dimerization is a prerequisite for the activation of the kinase. Kinase activation is visualized as tyrosine phosphorylation of the receptor molecules, which occurs between the dimerized receptor molecules (transphosphorylation). In conjunction with dimerization and kinase activation, the receptor molecules undergo conformational changes, which allow a basal kinase activity to phosphorylate a critical tyrosine residue, thereby "unlocking" the kinase, leading to full enzymatic activity directed toward other tyrosine residues in the receptor molecules as well as other substrates for the kinase. Expression of both receptors and each of the four PDGFs is under independent control, giving the PDGF/PDGFR system a high flexibility. Different cell types vary greatly in the ratio of PDGF isoforms and PDGFRs expressed. Different external stimuli such as inflammation, embryonic development or differentiation modulate cellular receptor expression allowing binding of some PDGFs but not others. Additionally, some cells display only one of the PDGFR isoforms while other cells express both isoforms, simultaneously or separately.
Interaction with signal transduction molecules
Tyrosine phosphorylation sites in growth factor receptors serve two major purposes: to control the state of activity of the kinase and to create binding sites for downstream signal transduction molecules, which in many cases also are substrates for the kinase. The second part of the tyrosine kinase domain in the PDGFβ receptor is phosphorylated at Tyr-857, and mutant receptors carrying phenylalanine at this position have reduced kinase activity. Tyr-857 has therefore been assigned a role in positive regulation of kinase activity. Sites of tyrosine phosphorylation involved in binding signal transduction molecules have been identified in the juxtamembrane domain, the kinase insert, and in the C-terminal tail in the PDGFβ receptor. The phosphorylated tyrosine residue and in general three adjacent C-terminal amino acid residues form specific binding sites for signal transduction molecules. Binding to these sites involves a common conserved stretches, denoted the Src homology (SH) 2 domain and/or Phosphotyrosine Binding Domains (PTB). The specificity of these interactions appears to be very high, since mutant receptors carrying phenylalanine residues in one or several of the different phosphorylation sites generally lack the capacity to bind the targeted signal transduction molecule. The signal transduction molecules are either equipped with different enzymatic activities, or they are adaptor molecules, which in some but not all cases are found in complexes with subunits that carry a catalytic activity. Upon interaction with the activated receptor, the catalytic activities become up-regulated, through tyrosine phosphorylation or other mechanisms, generating a signal that may be unique for each type of signal transduction molecule.
Examination of the different signalling cascades, induced by RTKs, established Ras/mitogen-activated protein kinase (MAPK),PI-3 kinase and phospholipase-γ (PLCγ) pathways as key downstream mediators of the PDGFR signalling.
MAPK pathway
The adaptor protein Grb2 forms a complex with Sos by the Grb2 SH3 domain. Grb2 (or) the Grb2/Sos complex is recruited to the membrane by the Grb2 SH2 domain binding to activated PDGFR-bound Syp/PTP1D (Also known as PTPN11, a cytosolic PTP), thereby allowing interaction with Ras and the exchange of GDP for GTP on Ras. Whereas the interaction between Grb2 and PDGFR occurs through interaction with the Syp/PTP1D protein, Grb2 binds to activated EGFR through Shc, another adaptor protein that forms a complex with many receptors via its PTB domain. (Schlessinger, J. SH2/SH3 Signaling Proteins. Curr. Op. Gen. Dev. 1994, 4: 25-30.) Once activated, Ras interacts with several proteins, namely Raf. Activated Raf stimulates MAPK-kinase (MAPKK or MEK) by phosphorylating a Ser residue in its activation loop. MAPKK then phosphorylates MAPK (ERK1/2) on T and Y residues at the activation-loop leading to its activation. Activated MAPK phosphorylates a variety of cytoplasmic substrates, as well as transcription factors, when translocated into the nucleus. MAPK family members have been found to regulate various biological functions by phosphorylation of particular target molecules (such as transcription factors, other kinases etc.) located in cell membrane, cytoplasm and nucleus, and thus contribute to the regulation of different cellular processes such as cell proliferation, differentiation, apoptosis and immunoresponses.
PI3K pathway
The class IA phospholipid kinase, PI-3 kinase, is activated by the majority of RTKs. Similarly to other SH2 domain-containing proteins, PI-3 kinase forms a complex with PY sites on activated receptors. The main function of PI3K activation is the generation of PIP3, which functions as a second messenger to activate downstream tyrosine kinases Btk and Itk, the Ser/Thr kinases PDK1 and Akt (PKB). The major biological functions of Akt activation can be classified into three categories – survival,proliferation and cell growth. Akt is also known to be implicated in several cancers, particularly breast. PLCγ is immediately recruited by an activated RTK through the binding of its SH2 domains to phosphotyrosine sites of the receptor. After activation, PLCγ hydrolyses its substrate PtdIns(4,5)P2 and forms two second messengers, diacylglycerol and Ins(1,4,5)P3. Ins(1,4,5)P3 stimulates the release of Ca 2+ from intracellular supplies. Ca 2+ then binds to calmodulin, which subsequently activates a family of calmodulindependent protein kinases (CamKs). In addition, both diacylglycerol and Ca 2+ activate members of the PKC family. The second messengers generated by PtdIns(4,5)P2 hydrolysis stimulate a variety of intracellular processes such as proliferation,angiogenesis, cell motility.
See also
- Receptor tyrosine kinase
- PDGF
- Imatinib
- PDGFRB
- Crenolanib (CP-868,596-26)
References
- ^ PDB 3MJG; Shima AHR, Liua H, Fociaa PJ, Chena X, Linb PC, He X. (2010). "Structures of a platelet-derived growth factor/propeptide complex and a platelet-derived growth factor/receptor complex". PNAS 107 (25): 11307–12. doi:10.1073/pnas.1000806107. PMC 2895058. PMID 20534510. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2895058.; rendered using PyMOL.
- ^ Williams LT (March 1989). "Signal transduction by the platelet-derived growth factor receptor". Science 243 (4898): 1564–70. doi:10.1126/science.2538922. PMID 2538922.
- ^ Heldin CH, Westermark B (April 1989). "Platelet-derived growth factor: three isoforms and two receptor types". Trends Genet. 5 (4): 108–11. doi:10.1016/0168-9525(89)90040-1. PMID 2543106.
- ^ Heldin CH, Ostman A, Eriksson A, Siegbahn A, Claesson-Welsh L, Westermark B (March 1992). "Platelet-derived growth factor: isoform-specific signalling via heterodimeric or homodimeric receptor complexes". Kidney Int. 41 (3): 571–4. doi:10.1038/ki.1992.84. PMID 1315403.
External links
Protein kinases: tyrosine kinases (EC 2.7.10) Receptor tyrosine kinases (EC 2.7.10.1) Insulin receptor familyPDGF receptor familyFGF receptor familyVEGF receptors familyHGF receptor familyTrk receptor familyEPH receptor familyLTK receptor familyLTK · ALKTIE receptor familyROR receptor familyROR1 · ROR2DDR receptor familyPTK7 receptor familyRYK receptor familyMuSK receptor familyROS receptor familyAATYK receptor familyAXL receptor familyRET receptor familyuncatagorisedNon-receptor tyrosine kinases (EC 2.7.10.2) ABL familyACK familyACK1 · TNK1CSK familyFAK familyFES familyFRK familyJAK familySRC-A familySRC-B familyTEC familySYK familyB enzm: 1.1/2/3/4/5/6/7/8/10/11/13/14/15-18, 2.1/2/3/4/5/6/7/8, 2.7.10, 2.7.11-12, 3.1/2/3/4/5/6/7, 3.1.3.48, 3.4.21/22/23/24, 4.1/2/3/4/5/6, 5.1/2/3/4/99, 6.1-3/4/5-6 Receptors: growth factor receptors Type I cytokine receptor Receptor protein serine/threonine kinase Receptor tyrosine kinase Fibroblast growth factor (1, 2, 3, 4)
Nerve growth factors: high affinity Trk (TrkA, TrkB, TrkC)
Somatomedin (Insulin-like growth factor 1)
VEGF (1, 2, 3)Tumor necrosis factor receptor Ig superfamily Platelet-derived growth factor (A, B)
Stem cell factorOther/ungrouped B trdu: iter (nrpl/grfl/cytl/horl), csrc (lgic, enzr, gprc, igsr, intg, nrpr/grfr/cytr), itra (adap, gbpr, mapk), calc, lipd; path (hedp, wntp, tgfp+mapp, notp, jakp, fsap, hipp, tlrp) Categories:- Genes on chromosome 4
- Genes on chromosome 5
- Growth factors
- Membrane biology
- Signal transduction
Wikimedia Foundation. 2010.
