- Varenicline
-
Varenicline 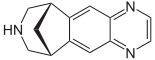
Systematic (IUPAC) name 7,8,9,10-tetrahydro- 6,10-methano- 6H-pyrazino (2,3-h)(3) benzazepine Clinical data Trade names Chantix AHFS/Drugs.com monograph MedlinePlus a606024 Licence data EMA:Link, US FDA:link Pregnancy cat. ?(US) Legal status ℞ Prescription only Routes Oral Pharmacokinetic data Protein binding <20% Metabolism Limited (<10%) Half-life 24 hours Excretion Renal (81–92%) Identifiers CAS number 249296-44-4 375815-87-5 ATC code N07BA03 PubChem CID 5310966 DrugBank DB01273 ChemSpider 4470510 
UNII W6HS99O8ZO 
ChEMBL CHEMBL1076903 
Chemical data Formula C13H13N3 Mol. mass 211.267 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Varenicline (trade name Chantix in the USA and Champix in Canada, Europe and other countries, marketed by Pfizer, usually in the form of varenicline tartrate), is a prescription medication used to treat smoking addiction. Varenicline stimulates nicotine receptors more weakly than nicotine does, that is, it is a nicotinic receptor partial agonist. In this respect it is similar to cytisine and different from the nicotinic antagonist, bupropion, and nicotine replacement therapies (NRTs) like nicotine patches and nicotine gum. As a partial agonist it both reduces cravings for and decreases the pleasurable effects of cigarettes and other tobacco products. Through these mechanisms it can assist some patients to quit smoking.
Contents
History
Varenicline was discovered at Pfizer through the research aimed at modifying the structure of cytisine.[1][2][3]
Varenicline received a "priority review" by the U.S. Food and Drug Administration (FDA) in February 2006, shortening the usual 10-month review period to 6 months because of its demonstrated effectiveness in clinical trials and perceived lack of safety issues.[4] The agency's approval of the drug came on May 11, 2006.[5] August 1, 2006, varenicline was made available for sale in the United States and on September 29, 2006, was approved for sale in the European Union.[6]
Indications
Varenicline is indicated for smoking cessation. It is more effective than NRTs and nicotine agonists.[7][8] In a 2006 randomized controlled trial sponsored by Pfizer, after one year the rate of continuous abstinence was 10% for placebo, 15% for bupropion and 23% for varenicline.[7] In a 2009 meta-analysis of 101 studies funded by Pfizer, varenicline was found to be more effective than bupropion (odds ratio 1.40) and NRTs (odds ratio 1.56).[8]
A Cochrane systematic review concluded that both varenicline and bupropion improved smoking cessation. More people quit with varenicline than with bupropion, but the difference was not statistically significant.[9]
The FDA has approved its use for twelve weeks. If smoking cessation has been achieved it may be continued for another twelve weeks.[5]
Varenicline has not been tested in those under 18 years old or pregnant women and therefore is not recommended for use by these groups.
Mechanism of action
Varenicline is a partial agonist of the α4β2 subtype of the nicotinic acetylcholine receptor. In addition it acts on α3β4 and weakly on α3β2 and α6-containing receptors. A full agonism was displayed on α7-receptors.[10]
Acting as a partial agonist varenicline binds to, and partially stimulates, the α4β2 receptor without producing a full effect like nicotine. Thus varenicline does not greatly increase the downstream release of dopamine. Due to its competitive binding on these receptors, varenicline blocks the ability of nicotine to bind and stimulate the mesolimbic dopamine system, akin to the action of buprenorphine in the treatment of opioid addiction.[11]
Pharmacokinetics
Most of the active compound is excreted renally (92–93%). A small proportion is glucuronidated, oxidated, N-formylated or conjugated to a hexose.[12] The elimination half-life is about 24 hours.
Side effects
Common
Nausea occurs commonly in people taking varenicline. Other less common side effects include headache, difficulty sleeping, and abnormal dreams. Rare side effects reported by people taking varenicline compared to placebo include change in taste, vomiting, abdominal pain, flatulence, and constipation. In a recent meta-analysis paper by Leung et al, it has been estimated that for every 5 subjects taking varenicline at maintenance does (1mg twice daily), there will be an event of nausea, and for every 24 and 35 treated subjects, there will be an event of constipation and flatulence respectively. Gastrointestinal side-effects are important factors compromising the compliance of varenicline.[13] [14]
Depression and suicide
In November 2007, the FDA announced it had received post-marketing reports that patients using varenicline for smoking cessation had experienced several serious symptoms, including suicidal ideation and occasional suicidal behavior, erratic behavior, and drowsiness. On February 1, 2008 the FDA issued an alert to further clarify its findings, noting that "it appears increasingly likely that there is an association between Chantix and serious neuropsychiatric symptoms." It is unknown whether the psychiatric symptoms are related to the drug or to nicotine withdrawal symptoms, although not all patients had stopped smoking. The FDA also recommended that health care professionals and patients watch for behavioral and mood changes.[15] In May 2008, Pfizer updated the safety information associated with varenicline, noting that "some patients have reported changes in behavior, agitation, depressed mood, suicidal thoughts or actions." [16]
As of July 1, 2009, the US Food and Drug Administration requires Chantix (varenicline) to carry a black box warning, the agency's strongest safety warning, due to public reports of side effects including depression, suicidal thoughts, and suicidal actions.[17]
Cardiovascular Disease
On June 16, 2011, the FDA issued a safety announcement that Chantix may be associated with a "a small, increased risk of certain cardiovascular adverse events in patients who have cardiovascular disease."[18]
On July 4, 2011, four scientists published a review of double-blind studies in the Canadian Medical Association Journal. They found that varenicline has increased risk of serious adverse cardiovascular events compared with placebo.[19]
Controversy
- The Institute for Safe Medication Practices (ISMP) conducted an analysis of post-marketing adverse effects reports received by the FDA. According to this analysis, in the fourth quarter of 2007 varenicline accounted for more reports of serious side effects than any other drug. Suicidal acts and ideation, psychosis, and hostility or aggression, including homicidal ideation, were the most prominent psychiatric side effects. Multiple reports suggested that varenicline may be related to the loss of glycemic control and new onset of diabetes, heart rhythm disturbances, skin reactions, vision disturbances, seizures, abnormal muscle spasms and other movement disorders. ISMP noted that the reports do not establish causality and only identify potential causes and concluded that further research and a priority review of the data by the FDA is necessary.[20]
- On Thursday, May 22, 2008, The New York Times reported that the U.S. Federal Aviation Administration had announced the day before a ban on the use of varenicline for both pilots and air traffic controllers, due to concerns with possible adverse neuropsychiatric effects which could be detrimental to public safety.[21]
- On Sunday, May 25, 2008, the Los Angeles Times reported that over 2 dozen traffic accidents had been linked to varenicline and reported to the FDA. Warnings had previously been issued by Pfizer regarding the risks of varenicline while driving.[22]
- On Tuesday, June 17, 2008, The Washington Times reported on its Front Page that the United States Department of Veterans Affairs was testing varenicline on war veterans with posttraumatic stress disorder without properly warning them of the side effects, and that in one case a veteran was almost killed when he had a psychotic episode and threatened police officers.[23]
- On October 23, 2008, the Institute for Safe Medication Practices issued an analysis of prescription drug-related injuries reported to the FDA during the first quarter of 2008. According to the report, varenicline had more reported incidents than any other drug, with 1001 new cases of adverse effects and 50 more deaths reported. (Heparin, the drug with the second highest number of injury reports, had 779 new cases, most of which were connected to a contaminant inadvertently introduced into the drug in early 2008). In comparison, the ISMP reported that in the first quarter of 2008 there were 17 serious injury reports for nicotine-replacement products, and 44 reports for bupropion (sold as Zyban as a smoking cessation medication). Varenicline did not rank in the ten drugs with the most related deaths, but did rank first in reports of suicide or self-injury, with 228 reports citing these effects. The ISMP noted that the high number of varenicline-related injury reports may be related to the publicity surrounding the medication's potential side effects.[24]
- On January 15, 2009, the Institute for Safe Medication Practices issued its analysis of prescription drug-related injuries reported to the FDA during the second quarter of 2008. During this period there were 910 newly-reported cases of serious injury attributed to varenicline, including 38 deaths. In addition to the above-reported psychiatric effects, the report noted increasing evidence linking varenicline to "potentially life-threatening allergic reactions." According to the report, varenicline had the second-highest number of new injury reports during this quarter. By comparison, digoxin had the highest number, with 1882 injury reports and 650 associated deaths, the majority of which were linked to a manufacturing quality control problem and subsequent recall of the Digitek brand of digoxin.[25]
- On February 4, 2009, Health Canada announced that it had received more than 800 complaints from Canadian users, many of them reporting mood swings, depression or suicidal thoughts.[26]
- A cohort study published in November 2009 analyzed medical records of 80,660 persons attempting to quit smoking (10,973 of which were using varenicline) and found no evidence of an increased risk of depression, self-harm and suicidality, although a small increase could not be ruled out on statistical grounds.[27]
- A study conducted by Group Health Center for Health Studies, SRI International, and Free & Clear, Inc., published in the Journal of General Internal Medicine on February 24, 2009, concluded that people with a history of depression are not qualitatively more susceptible to the reported psychiatric side effects of varenicline than people with no history of depression.[28]
- The Therapeutic Goods Administration of Australia has received more than 1000 reports of adverse events related to varenicline as of May 2010, with 67% of these describing psychiatric symptoms such as depression, aggression and anxiety.[29]
- On June 3, 2010, Health Canada also announced changes to the Canadian Product Monograph that include changes in mood or behaviour (such as depressed mood, agitation, aggression, hostility, thoughts of self-harm or harm towards others); serious allergic reactions (such as swelling of the face, lips, gums, tongue and throat that can cause trouble breathing) and skin reactions (such as rash, swelling, redness, and peeling of the skin); neuropsychiatric side-effects in patients taking varenicline with or without a history of psychiatric disorder; drinking alcohol increasing the risk of experiencing neuropsychiatric side effects; and side-effects such as sleepiness, dizziness, loss of consciousness, seizures, or difficulty concentrating. Health Canada advised those taking varenicline not engage in potentially hazardous activities, such as driving a car or operating dangerous machinery until they know how they may be affected by varenicline.[30]
- In May 2011, it was revealed that Pfizer had submitted 589 varenicline-related adverse effects reports to the FDA through "improper channels." These reports dated back through 2007 and included 150 completed suicides (more than twice the number previously reported).[31]
- On May 31, 2011, French Health Minister Xavier Bertrand announced that varenicline prescriptions would no longer be subsidized by the government health insurance, due to questions about its safety.[32]
- In 26 July 2011, The European Medicines Agency has announced that the slightly increased risk of cardiovascular events associated with varenicline (Champix) reported in a recent meta-analysis[33] does not outweigh the benefits of the drug in helping people to stop smoking.[34]
See also
- Drug Discovery and Development: Nicotinic Acetylcholine Receptor Agonists
References
- ^ Coe JW, Brooks PR, Vetelino MG et al. (2005). "Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation". J. Med. Chem. 48 (10): 3474–3477. doi:10.1021/jm050069n. PMID 15887955.
- ^ Schwartz JL (1979). "Review and evaluation of methods of smoking cessation, 1969–77. Summary of a monograph". Public Health Rep 94 (6): 558–63. PMC 1431736. PMID 515342. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1431736.
- ^ Etter JF (2006). "Cytisine for smoking cessation: a literature review and a meta-analysis". Arch. Intern. Med. 166 (15): 1553–1559. doi:10.1001/archinte.166.15.1553. PMID 16908787. http://archinte.ama-assn.org/cgi/content/full/166/15/1553.
- ^ Kuehn BM (2006). "FDA speeds smoking cessation drug review". JAMA 295 (6): 614–614. doi:10.1001/jama.295.6.614. PMID 16467225.
- ^ a b U.S. Food and Drug Administration. FDA Approves Novel Medication for Smoking Cessation. Press release, 11 May 2006.
- ^ European Medicines Agency (2011-01-28). "EPAR summary for the public. Champix varenicline". London. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000699/human_med_000696.jsp. Retrieved 2011-02-14.
- ^ a b Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR (2006). "Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial". JAMA 296 (1): 56–63. doi:10.1001/jama.296.1.56. PMID 16820547. http://jama.ama-assn.org/content/296/1/56.full.
- ^ a b Mills EJ, Wu P, Spurden D, Ebbert JO, Wilson K (2009). "Efficacy of pharmacotherapies for short-term smoking abstinance: a systematic review and meta-analysis". Harm Reduct J 6: 25. doi:10.1186/1477-7517-6-25. PMC 2760513. PMID 19761618. http://www.biomedcentral.com/content/pdf/1477-7517-6-25.pdf.
- ^ "Nicotine receptor partial agonists for smoking cessation". Cochrane Database of Systematic Reviews (2). 16 FEB 2011. doi:DOI: 10.1002/14651858.CD006103.pub. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD006103.pub5/abstract.
- ^ Mihalak KB, Carroll FI, Luetje CW (2006). "Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors". Mol. Pharmacol. 70 (3): 801–805. doi:10.1124/mol.106.025130. PMID 16766716.
- ^ Rollema H, Chambers LK, Coe JW et al. (March 2007). "Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid". Neuropharmacology 52 (3): 985–994. doi:10.1016/j.neuropharm.2006.10.016. PMID 17157884.
- ^ Obach, RS; Reed-Hagen, AE; Krueger, SS; Obach, BJ; O'Connell, TN; Zandi, KS; Miller, S; Coe, JW (2006). "Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro". Drug metabolism and disposition: the biological fate of chemicals 34 (1): 121–130. doi:10.1124/dmd.105.006767. PMID 16221753.
- ^ Leung, LK; Patafio, FM, Rosser, WW (2011 Sep 28). "Gastrointestinal adverse effects of varenicline at maintenance dose: a meta-analysis.". BMC clinical pharmacology 11 (1): 15. doi:10.1186/1472-6904-11-15. PMID 21955317. http://www.biomedcentral.com/1472-6904/11/15.
- ^ American Cancer Society. "Cancer Drug Guide: Varenicline". http://www.cancer.org/docroot/CDG/content/CDG_Varenicline.asp. Retrieved 2008-01-19.
- ^ "Early Communication About an Ongoing Safety Review: Varenicline (marketed as Chantix)". United States Food and Drug Administration. November 20 2007. Archived from the original on 2007-12-05. http://web.archive.org/web/20071205222932/http://www.fda.gov/cder/drug/early_comm/varenicline.htm. Retrieved 2007-11-21.
- ^ "Important Information about Chantix". http://www.chantix.com/content/important_info_about_chantix.jsp. Retrieved 2008-05.
- ^ FDA. "Public Health Advisory: FDA Requires New Boxed Warnings for the Smoking Cessation Drugs Chantix and Zyban". http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm169988.htm. Retrieved 2009-07-01.
- ^ "FDA Drug Safety Communication: Chantix (varenicline) may increase the risk of certain cardiovascular adverse events in patients with cardiovascular disease". 2011-06-16. http://www.fda.gov/Drugs/DrugSafety/ucm259161.htm.
- ^ Cohn, Meredith (July 4, 2011). "Chantix may cause more heart attacks than previously thought" (PDF). The Baltimore Sun (Tribune Company). http://www.cmaj.ca/content/early/2011/07/04/cmaj.110218.full.pdf+html. Retrieved July 4, 2011. and Singh S, Loke Y, Spangler J, and Furberg C (July 4, 2011). "Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis" (PDF). Canadian Medical Association Journal (CMAJ). doi:10.1503/cmaj.110218. http://www.cmaj.ca/content/early/2011/07/04/cmaj.110218.full.pdf+html. Retrieved July 4, 2011.
- ^ Institute for Safe Medication Practices. "Strong Safety Signal Seen for New Varenicline Risks". http://www.ismp.org/quarterwatch/chantixReport.asp.
- ^ Saul, Stephanie (2008-05-22). "F.A.A. Bans Antismoking Drug, Citing Side Effects". New York Times. http://www.nytimes.com/2008/05/22/business/22drug.html. Retrieved 2008-05-22.
- ^ Alonso-Zaldivar, Ricardo (2008-05-25). "Drug taken to stop smoking is linked to traffic mishaps". Los Angeles Times. http://www.latimes.com/news/nationworld/nation/la-na-smokedrug25-2008may25,0,6554950.story. Retrieved 2008-05-25.
- ^ Hudson, Audrey (2008-06-17). "VA Testing Drugs on War Veterans". The Washington Times: p. A1.
- ^ Institute for Safe Medication Practices. "Quarter Watch: 2008 Quarter 1". http://www.ismp.org/QuarterWatch/2008Q1.pdf. Retrieved 2008-10-23.
- ^ Institute for Safe Medication Practices. "Quarter Watch: 2008 Quarter 2". http://www.ismp.org/quarterwatch/200901.pdf. Retrieved 2009-01-15.
- ^ "Over 800 complaints on quit-smoking aid reported to Health Canada". CBC. February 4, 22009. http://www.cbc.ca/health/story/2009/02/04/champix.html. Retrieved 2009-02-09.
- ^ Gunnell, D; Irvine, D; Wise, L; Davies, C; Martin, RM (2009). "Varenicline and suicidal behaviour: A cohort study based on data from the General Practice Research Database". BMJ (Clinical research ed.) 339: b3805–b3805. doi:10.1136/bmj.b3805. PMC 2755726. PMID 19797344. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2755726.
- ^ McClure JB, Swan GE, Jack L et al. (May 2009). "Mood, side-effects and smoking outcomes among persons with and without probable lifetime depression taking varenicline". J Gen Intern Med 24 (5): 563–569. doi:10.1007/s11606-009-0926-8. PMC 2669860. PMID 19238488. http://www.springerlink.com/content/77207452k3822r3v/fulltext.html.
- ^ Elijah, Jennifer. "Varenicline (Champix): an update". Australian Prescriber (Australia) (33). http://www.australianprescriber.com/magazine/33/4/120/3. Retrieved August 12, 2010.
- ^ "CHAMPIX (varenicline tartrate) — Changes to the Canadian Product Monograph — For the Public". Health Canada. June 3, 2010. http://www.hc-sc.gc.ca/dhp-mps/medeff/advisories-avis/public/_2010/champix_2_pc-cp-eng.php. Retrieved 2010-06-03.
- ^ Institute for Safe Medication Practices. "Quarter Watch: 2010 Quarter 3". http://ismp.org/Newsletters/acutecare/articles/20110519.asp.
- ^ Lee Howard. "France Stops Paying for Chantix". http://www.theday.com/article/20110601/BIZ02/306019907/-1/BIZ.
- ^ "Varenicline raises risk of heart problems, analysis indicates". BMJ. http://www.bmj.com/content/343/bmj.d4428.extract.
- ^ "Benefits of varenicline outweigh risks". BMJ. http://www.bmj.com/content/343/bmj.d4728.short?rss=1&utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+bmj%2Frecent+%28Latest+from+BMJ%29.
External links
Stimulants (N06B) Adamantanes Adaphenoxate • Adapromine • Amantadine • Bromantane • Chlodantane • Gludantane • Memantine • Midantane
Adenosine antagonists 8-Chlorotheophylline • 8-Cyclopentyltheophylline • 8-Phenyltheophylline • Aminophylline • Caffeine • CGS-15943 • Dimethazan • Paraxanthine • SCH-58261 • Theobromine • TheophyllineAlkylamines Arylcyclohexylamines Benocyclidine • Dieticyclidine • Esketamine • Eticyclidine • Gacyclidine • Ketamine • Phencyclamine • Phencyclidine • Rolicyclidine • Tenocyclidine • Tiletamine
Benzazepines 6-Br-APB • SKF-77434 • SKF-81297 • SKF-82958
Cholinergics A-84543 • A-366,833 • ABT-202 • ABT-418 • AR-R17779 • Altinicline • Anabasine • Arecoline • Cotinine • Cytisine • Dianicline • Epibatidine • Epiboxidine • GTS-21 • Ispronicline • Nicotine • PHA-543,613 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • SIB-1553A • SSR-180,711 • TC-1698 • TC-1827 • TC-2216 • TC-5619 • Tebanicline • UB-165 • Varenicline • WAY-317,538
Convulsants Anatoxin-a • Bicuculline • DMCM • Flurothyl • Gabazine • Pentetrazol • Picrotoxin • Strychnine • Thujone
Eugeroics Adrafinil • Armodafinil • CRL-40941 • Modafinil
Oxazolines 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone
Phenethylamines 1-(4-Methylphenyl)-2-aminobutane • 1-Phenyl-2-(piperidin-1-yl)pentan-3-one • 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane • 2-Fluoroamphetamine • 2-Fluoromethamphetamine • 2-OH-PEA • 2-Phenyl-3-aminobutane • 2-Phenyl-3-methylaminobutane • 2,3-MDA • 3-Fluoroamphetamine • 3-Fluoroethamphetamine • 3-Fluoromethcathinone • 3-Methoxyamphetamine • 3-Methylamphetamine • 3,4-DMMC • 4-BMC • 4-Ethylamphetamine • 4-FA • 4-FMA • 4-MA • 4-MMA • 4-MTA • 6-FNE • Alfetamine • α-Ethylphenethylamine • Amfecloral • Amfepentorex • Amfepramone • Amidephrine • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Arbutamine • β-Methylphenethylamine • β-Phenylmethamphetamine • Benfluorex • Benzedrone • Benzphetamine • BDB (J) • BOH (Hydroxy-J) • BPAP • Buphedrone • Bupropion (Amfebutamone) • Butylone • Cathine • Cathinone • Chlorphentermine • Cinnamedrine • Clenbuterol • Clobenzorex • Cloforex • Clortermine • D-Deprenyl • Denopamine • Dimethoxyamphetamine • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Dobutamine • DOPA (Dextrodopa, Levodopa) • Dopamine • Dopexamine • Droxidopa • EBDB (Ethyl-J) • Ephedrine • Epinephrine (Adrenaline) • Epinine (Deoxyepinephrine) • Etafedrine • Ethcathinone (Ethylpropion) • Ethylamphetamine (Etilamfetamine) • Ethylnorepinephrine (Butanefrine) • Ethylone • Etilefrine • Famprofazone • Fenbutrazate • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenmetramide • Fenproporex • Flephedrone • Fludorex • Furfenorex • Gepefrine • HMMA • Hordenine • Ibopamine • IMP • Indanylamphetamine • Isoetarine • Isoethcathinone • Isoprenaline (Isoproterenol) • L-Deprenyl (Selegiline) • Lefetamine • Lisdexamfetamine • Lophophine (Homomyristicylamine) • Manifaxine • MBDB (Methyl-J; "Eden") • MDA (Tenamfetamine) • MDBU • MDEA ("Eve") • MDMA ("Ecstasy", "Adam") • MDMPEA (Homarylamine) • MDOH • MDPR • MDPEA (Homopiperonylamine) • Mefenorex • Mephedrone • Mephentermine • Metanephrine • Metaraminol • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methoxamine • Methoxyphenamine • MMA • Methcathinone (Methylpropion) • Methedrone • Methoxyphenamine • Methylone • MMDA • MMDMA • MMMA • Morazone • N-Benzyl-1-phenethylamine • N,N-Dimethylphenethylamine • Naphthylamphetamine • Nisoxetine • Norepinephrine (Noradrenaline) • Norfenefrine • Norfenfluramine • Normetanephrine • Octopamine • Orciprenaline • Ortetamine • Oxilofrine • Paredrine (Norpholedrine, Oxamphetamine, Mycadrine) • PBA • PCA • PHA • Pargyline • Pentorex (Phenpentermine) • Pentylone • Phendimetrazine • Phenmetrazine • Phenpromethamine • Phentermine • Phenylalanine • Phenylephrine (Neosynephrine) • Phenylpropanolamine • Pholedrine • PIA • PMA • PMEA • PMMA • PPAP • Prenylamine • Propylamphetamine • Pseudoephedrine • Radafaxine • Ropinirole • Salbutamol (Albuterol; Levosalbutamol) • Sibutramine • Synephrine (Oxedrine) • Theodrenaline • Tiflorex (Flutiorex) • Tranylcypromine • Tyramine • Tyrosine • Xamoterol • Xylopropamine • Zylofuramine
Piperazines Piperidines 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine • 1-(3,4-Dichlorophenyl)-1-(piperidin-2-yl)butane • 2-Benzylpiperidine • 2-Methyl-3-phenylpiperidine • 3,4-Dichloromethylphenidate • 4-Benzylpiperidine • 4-Methylmethylphenidate • Desoxypipradrol • Difemetorex • Diphenylpyraline • Ethylphenidate • Methylnaphthidate • Methylphenidate (Dexmethylphenidate) • N-Methyl-3β-propyl-4β-(4-chlorophenyl)piperidine • Nocaine • Phacetoperane • Pipradrol • SCH-5472
Pyrrolidines 2-Diphenylmethylpyrrolidine • α-PPP • α-PBP • α-PVP • Diphenylprolinol • MDPPP • MDPBP • MDPV • MPBP • MPHP • MPPP • MOPPP • Naphyrone • PEP • Prolintane • Pyrovalerone
Tropanes 3-CPMT • 3'-Chloro-3α-(diphenylmethoxy)tropane • 3-Pseudotropyl-4-fluorobenzoate • 4'-Fluorococaine • AHN-1055 • Altropane (IACFT) • Brasofensine • CFT (WIN 35,428) • β-CIT (RTI-55) • Cocaethylene • Cocaine • Dichloropane (RTI-111) • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Norcocaine • PIT • PTT • RTI-31 • RTI-32 • RTI-51 • RTI-105 • RTI-112 • RTI-113 • RTI-117 • RTI-120 • RTI-121 (IPCIT) • RTI-126 • RTI-150 • RTI-154 • RTI-171 • RTI-177 • RTI-183 • RTI-193 • RTI-194 • RTI-199 • RTI-202 • RTI-204 • RTI-229 • RTI-241 • RTI-336 • RTI-354 • RTI-371 • RTI-386 • Salicylmethylecgonine • Tesofensine • Troparil (β-CPT, WIN 35,065-2) • Tropoxane • WF-23 • WF-33 • WF-60
Others 1-(Thiophen-2-yl)-2-aminopropane • 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-MDP • 2-Phenylcyclohexylamine • 2-Phenyl-3,6-dimethylmorpholine • 3-Benzhydrylmorpholine • 3,3-Diphenylcyclobutanamine • 5-(2-Aminopropyl)indole • 5-Iodo-2-aminoindane • AL-1095 • Amfonelic acid • Amineptine • Amiphenazole • Atipamezole • Atomoxetine (Tomoxetine) • Bemegride • Benzydamine • BTQ • BTS 74,398 • Carphedon • Ciclazindol • Cilobamine • Clofenciclan • Cropropamide • Crotetamide • Cypenamine • D-161 • Diclofensine • Dimethocaine • Efaroxan • Etamivan • EXP-561 • Fencamfamine • Fenpentadiol • Feprosidnine • G-130 • Gamfexine • Gilutensin • GSK1360707F • GYKI-52895 • Hexacyclonate • Idazoxan • Indanorex • Indatraline • JNJ-7925476 • JZ-IV-10 • Lazabemide • Leptacline • Levopropylhexedrine • Lomevactone • LR-5182 • Mazindol • Meclofenoxate • Medifoxamine • Mefexamide • Mesocarb • Methastyridone • Methiopropamine • N-Methyl-3-phenylnorbornan-2-amine • Nefopam • Nikethamide • Nomifensine • O-2172 • Oxaprotiline • Phthalimidopropiophenone • PNU-99,194 • Propylhexedrine • PRC200-SS • Rasagiline • Rauwolscine • Rubidium chloride • Setazindol • Tametraline • Tandamine • Trazium • UH-232 • Yohimbine
See also Sympathomimetic amines Nootropics (N06B) Acetylcholinesterases Ampakines CX-516 • CX-546 • CX-614 • CX-691 • CX-717 • IDRA-21 • LY-404,187 • LY-503,430 • PEPA • Sunifiram • UnifiramD1 Agonists Eugeroics GABAA α5 Inverse Agonists H3 Antagonists mACh Agonists Alvameline • Arecoline • Cevimeline • CI-1017 • Milameline • Sabcomeline • Talsaclidine • Tazomeline • XanomelinenACh Agonists AR-R17779 • Ispronicline • Nicotine • PNU-282,987 • SSR-180,711 • WAY-317,538Racetams Others Acetylcarnitine • Adafenoxate • Bifemelane • Bilobalide (Ginkgo Biloba) • Carbenoxolone • Cerlapirdine • Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine • Ensaculin • Fipexide • Idebenone • Indeloxazine • Latrepirdine • Leteprinim • Linopirdine • Meclofenoxate • Nizofenone • Pirisudanol • Pyritinol • S-17092 • Sulbutiamine • Taltirelin • Teniloxazine • Tricyanoaminopropene • VinpocetineAntiaddictives (N07B) Nicotine dependence/
(Nicotinic agonist)Nicotine • Dianicline • Varenicline • Lobeline • Mecamylamine • Scopolamine
NDRI (Bupropion) • AA (Clonidine) • CB1 (Surinabant)Alcohol dependence Opioid dependence Stimulant dependence Benzodiazepine dependence Cocaine dependence Sedative-Hypnotic dependence Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Smoking cessation
- Nicotinic agonists
- Pfizer
- Benzazepines
- Heterocyclic compounds (bridged-ring)
- Pyrazines
Wikimedia Foundation. 2010.
Look at other dictionaries:
Varénicline — Général Nom IUPAC 7,8,9,10 tétrahydro 6,10 méthano 6H pyrazino(2,3 h)(3)benzazépine No CAS … Wikipédia en Français
Varenicline — Varénicline Varénicline Général Nom IUPAC 7,8,9,10 tetrahydro 6,10 methano 6H pyrazino(2,3 h)(3)benzazepine N … Wikipédia en Français
varenicline — /və ren i klēn/ noun A drug used in the treatment of nicotine addiction … Useful english dictionary
varenicline — noun A particular pharmaceutical drug, an agonist for nicotine, used to aid tobacco smoking cessation. Farewell Zyban, hello Champix, Pfizers name for a new compound called varenicline, which is not an anti depressant but a ‘nicotine receptor… … Wiktionary
varenicline tartrate — va·ren·i·cline tar·trate (və renґĭ klēn) a partial agonist of one type of nicotinic receptor, used as an aid in smoking cessation; administered orally … Medical dictionary
varenicline tartrate — A drug used to help people stop smoking by acting the same way nicotine acts in the brain. It is a type of nicotine receptor partial agonist. Also called Chantix … English dictionary of cancer terms
249296-44-4 — Varénicline Varénicline Général Nom IUPAC 7,8,9,10 tetrahydro 6,10 methano 6H pyrazino(2,3 h)(3)benzazepine … Wikipédia en Français
C13H13N3 — Varénicline Varénicline Général Nom IUPAC 7,8,9,10 tetrahydro 6,10 methano 6H pyrazino(2,3 h)(3)benzazepine … Wikipédia en Français
Champix — Varénicline Varénicline Général Nom IUPAC 7,8,9,10 tetrahydro 6,10 methano 6H pyrazino(2,3 h)(3)benzazepine … Wikipédia en Français
Варениклин — (торговое название препарата Chantix в США и Champix в Европе и других странах) был создан фармацевтической компанией Pfizer. Первые продажи начались в 2006 году. Чампикс применяется в качестве средства против табакокурения у взрослых … Википедия
