- Cocaethylene
-
Cocaethylene 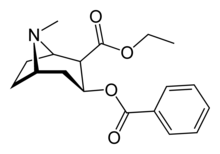
Systematic (IUPAC) name ethyl (2R,3S)-3-benzoyloxy-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate Clinical data Pregnancy cat. C Legal status CD (UK) Not scheduled specifically, could be prosecuted as an analogue of a scheduled substance. Dependence liability Moderate to High Routes Produced from ingestion of cocaine and ethanol Identifiers CAS number 529-38-4 ATC code None PubChem CID 65034 ChemSpider 559082 Synonyms benzoylecgonine ethyl ester, ethylbenzoylecgonine, Chemical data Formula C18H23NO4 Mol. mass 317.38 g/mol SMILES eMolecules & PubChem Cocaethylene (ethylbenzoylecgonine) is the ethyl ester of benzoylecgonine. It is structurally similar to cocaine, which is the methyl ester of benzoylecgonine. Cocaethylene is formed in vivo when cocaine and ethyl alcohol have been ingested simultaneously.[1]
Normally cocaine's metabolism produces two major and biologically inactive metabolites, benzoylecogonine and ecgonine methyl ester. Carboxylesterase is an important part of cocaine's metabolism because it acts as the catalyst for the hydrolysis of cocaine which produces the inactive metabolites. If ethanol is present during the metabolism of cocaine, a portion of the cocaine undergoes transesterification with ethanol, instead of undergoing hydrolysis with water, which results in cocaethylene.[2]
Cocaethylene is a recreational drug with stimulant, euphoriant, anorectic, sympathomimetic and local anesthetic properties. Three monoamine neurotransmitters known as serotonin (5-HT), norepinephrine (NE), and dopamine (DA) play an important role in cocaethylene's action. Cocaethylene increases the level of serotonergic, noradrenergic, and dopaminergic neurotransmission by inhibiting the action of the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT) which makes cocaethylene a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI).[Note 1]
Cocaethylene appears to, in most users, produce more euphoria and possess a longer duration of action than cocaine. Some studies suggest that it may be more cardiotoxic than cocaine. Cocaethylene is more potent than cocaine at binding to the dopamine transporter, however it is less potent at binding to the serotonin transporter and norepinephrine transporter.[3][4]
Contents
Notes
Footnotes
- ^ Serotonin-norepinephrine-dopamine reuptake inhibitors are also known as triple reuptake inhibitor which is abbreviated TRI
References
- ^ S. Casey Laizure, Timothy Mandrell, Naomi M. Gades, and Robert B. Parker (2003). "Cocaethylene Metabolism and Interaction with Cocaine and Ethanol: Role of Carboxylesterases". Drug Metabolism and Disposition 31 (1): 16–20. doi:10.1124/dmd.31.1.16. PMID 12485948.
- ^ Cocaethylene metabolism : interaction with cocaine and ethanol Cocaethylene metabolism and interaction with cocaine and ethanol: role of carboxylesterases by Laizure SC, Mandrell T, Gades NM, Parker RB (January 31st, 2003).
- ^ Jatlow, Peter; McCance, Elinore F.; Bradberry, Charles W.; Elsworth, John D.; Taylor, Jane R.; Roth, Robert H. (1996). noradrenaline transporter(NAT) 00026&type=abstract "Alcohol plus Cocaine: The Whole Is More Than the Sum of Its Parts". Therapeutic Drug Monitoring 18 (4): 460–4. doi:10.1097/00007691-199608000-00026. PMID 8857569. http://jousrnals.lww.com/drug-monitoring/pages/articleviewer.aspx?year=1996&issue=08000&article=the noradrenaline transporter(NAT) 00026&type=abstract.
- ^ M. Perez et al. (1994). "Cocaine and cocaethylene: microdialysis comparison of brain drug levels and effects on dopamine and serotonin". Psychopharmacology (Berl.) 116 (4): 428–32. doi:10.1111/j.1471-4159.1993.tb03305.x. PMID 8455033.
Further reading
- Cocaethylene: responding to combined alcohol and cocaine use
- M. J. Landry (1992). "An overview of cocaethylene, an alcohol-derived, psychoactive, cocaine metabolite.". J. Psychoactive Drugs 24 (3): 273–6. PMID 1432406. http://cocaine.org/cocaethylene.htm.
- Warning of extra heart dangers from mixing cocaine and alcohol
See also
- Cocaine
- Drug addiction
- Ethanol
- Ethylphenidate
- Euphoriants
- Methylvanillylecgonine
- Local anesthetics
- Recreational drugs
- Stimulants
- Tropanes
- Vin Mariani
Wikimedia Foundation. 2010.
