- Diphenylprolinol
-
Diphenylprolinol 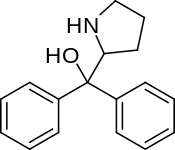
Systematic (IUPAC) name diphenyl(pyrrolidin-2-yl)methanol Clinical data Pregnancy cat. ? Legal status ? Identifiers CAS number 22348-32-9 ATC code None PubChem CID 204386 ChemSpider 21106295 Chemical data Formula C17H19NO Mol. mass 253.33886 g/mol - InChI=1S/C17H19NO/c19-17(16-12-7-13-18-16,14-8-3-1-4-9-14)15-10-5-2-6-11-15/h1-6,8-11,16,18-19H,7,12-13H2
Key:OGCGXUGBDJGFFY-UHFFFAOYSA-N
Diphenylprolinol (D2PM), or (R/S)-(±)-diphenyl-2-pyrrolidinyl-methanol, is a relatively mild norepinephrine-dopamine reuptake inhibitor which is used as a designer drug.[1][2]
Contents
Pharmacology
The dextrorotary (R)-(+)-enantiomer is the most pharmacologically active, although a variety of related derivatives have been studied.[3] Anecdotally, the dextrorotary enantiomer is orally active in the 2 mg - 5 mg range as larger doses e.g. 25 mg+ would prove fatal, with a duration of 5–8 hours. The effects are said to be similar to other NDRIs, producing mild stimulation (at least in relation to substances like methamphetamine or cocaine) which is productive for working or studying, but with relatively little euphoria. Similar drugs to diphenylprolinol include pipradrol, desoxypipradrol, and to a lesser extent, methylphenidate.
There have been indications from detailed reports posted on Internet forums dedicated to researching such compounds that diphenylprolinol may not be as benign as its mild effects seem to suggest.[citation needed] At higher doses and with more frequent administration, its effects on dopamine can become significant enough for binging and addiction to enter the picture. Several large overdoses requiring hospitalization have been reported, possibly related to addiction issues. It is advisable to exercise caution when administering this compound, even for occasional use, as most dopamine reuptake inhibitors are addictive.
Side effects including chest pain (suggestive of possible cardiovascular toxicity) have been seen following recreational use of diphenylprolinol, although it was combined with glaucine in a party pill product ("Head Candy" brand), thus making it impossible to say for certain which drug was responsible.[4]
Use in organic synthesis
In organic synthesis, diphenylprolinol can be used to prepare the chiral CBS catalyst, which is used for enantioselective reduction of prochiral ketones.[5] The catalyst is also useful in asymmetric Aldol reactions. Diphenylprolinol is condensed with a phenylboronic acid, or with borane (as shown below), to give the CBS catalyst, which complexes in situ with borane to give the active catalyst.[6]

See also
- 2-Diphenylmethylpyrrolidine
- Desoxypipradrol
- Pipradrol
- Prolinol
- Corey-Bakshi-Shibata reduction
- CBS catalyst
References
- ^ Wood DM et al, Detection of the novel recreational drug Diphenyl-2-pyrrolidinemethanol (D2PM) sold legally in combination with 1-Benzylpiperzaine (BZP). Clinical Toxicology 2008; 46:393.
- ^ Davies S. Drug Trends and New Designer Drugs. St George's University of London. 6th November 2008.
- ^ Paul F. Jackson et al. Pharmaceutical compositions and methods for treating compulsive disorders using pyrrolidine derivatives. US Patent 5295666
- ^ Lidder, S; Dargan, P; Sexton, M; Button, J; Ramsey, J; Holt, D; Wood, D (2008). "Cardiovascular toxicity associated with recreational use of diphenylprolinol (diphenyl-2-pyrrolidinemethanol D2PM)". Journal of medical toxicology : official journal of the American College of Medical Toxicology 4 (3): 167–9. PMID 18821489.
- ^ Corey, E. J.; Bakshi, R. K.; Shibata S., (1987). "Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines Mechanism and synthetic implications". J. Am. Chem. Soc. 109 (18): 5551. doi:10.1021/ja00252a056. ISSN 0002-7863.
- ^ Lohray, B. B., Bhushan, V. (1992). "Oxazaborolidines and Dioxaborolidines in Enantioselective Catalysis". Angew. Chem.-Int. Edit. Engl. 31 (6): 729–730. doi:10.1002/anie.199207291. ISSN 0570-0833.
External links
Stimulants (N06B) Adamantanes Adaphenoxate • Adapromine • Amantadine • Bromantane • Chlodantane • Gludantane • Memantine • Midantane
Adenosine antagonists 8-Chlorotheophylline • 8-Cyclopentyltheophylline • 8-Phenyltheophylline • Aminophylline • Caffeine • CGS-15943 • Dimethazan • Paraxanthine • SCH-58261 • Theobromine • TheophyllineAlkylamines Arylcyclohexylamines Benocyclidine • Dieticyclidine • Esketamine • Eticyclidine • Gacyclidine • Ketamine • Phencyclamine • Phencyclidine • Rolicyclidine • Tenocyclidine • Tiletamine
Benzazepines 6-Br-APB • SKF-77434 • SKF-81297 • SKF-82958
Cholinergics A-84543 • A-366,833 • ABT-202 • ABT-418 • AR-R17779 • Altinicline • Anabasine • Arecoline • Cotinine • Cytisine • Dianicline • Epibatidine • Epiboxidine • GTS-21 • Ispronicline • Nicotine • PHA-543,613 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • SIB-1553A • SSR-180,711 • TC-1698 • TC-1827 • TC-2216 • TC-5619 • Tebanicline • UB-165 • Varenicline • WAY-317,538
Convulsants Anatoxin-a • Bicuculline • DMCM • Flurothyl • Gabazine • Pentetrazol • Picrotoxin • Strychnine • Thujone
Eugeroics Adrafinil • Armodafinil • CRL-40941 • Modafinil
Oxazolines 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone
Phenethylamines 1-(4-Methylphenyl)-2-aminobutane • 1-Phenyl-2-(piperidin-1-yl)pentan-3-one • 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane • 2-Fluoroamphetamine • 2-Fluoromethamphetamine • 2-OH-PEA • 2-Phenyl-3-aminobutane • 2-Phenyl-3-methylaminobutane • 2,3-MDA • 3-Fluoroamphetamine • 3-Fluoroethamphetamine • 3-Fluoromethcathinone • 3-Methoxyamphetamine • 3-Methylamphetamine • 3,4-DMMC • 4-BMC • 4-Ethylamphetamine • 4-FA • 4-FMA • 4-MA • 4-MMA • 4-MTA • 6-FNE • Alfetamine • α-Ethylphenethylamine • Amfecloral • Amfepentorex • Amfepramone • Amidephrine • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Arbutamine • β-Methylphenethylamine • β-Phenylmethamphetamine • Benfluorex • Benzedrone • Benzphetamine • BDB (J) • BOH (Hydroxy-J) • BPAP • Buphedrone • Bupropion (Amfebutamone) • Butylone • Cathine • Cathinone • Chlorphentermine • Cinnamedrine • Clenbuterol • Clobenzorex • Cloforex • Clortermine • D-Deprenyl • Denopamine • Dimethoxyamphetamine • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Dobutamine • DOPA (Dextrodopa, Levodopa) • Dopamine • Dopexamine • Droxidopa • EBDB (Ethyl-J) • Ephedrine • Epinephrine (Adrenaline) • Epinine (Deoxyepinephrine) • Etafedrine • Ethcathinone (Ethylpropion) • Ethylamphetamine (Etilamfetamine) • Ethylnorepinephrine (Butanefrine) • Ethylone • Etilefrine • Famprofazone • Fenbutrazate • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenmetramide • Fenproporex • Flephedrone • Fludorex • Furfenorex • Gepefrine • HMMA • Hordenine • Ibopamine • IMP • Indanylamphetamine • Isoetarine • Isoethcathinone • Isoprenaline (Isoproterenol) • L-Deprenyl (Selegiline) • Lefetamine • Lisdexamfetamine • Lophophine (Homomyristicylamine) • Manifaxine • MBDB (Methyl-J; "Eden") • MDA (Tenamfetamine) • MDBU • MDEA ("Eve") • MDMA ("Ecstasy", "Adam") • MDMPEA (Homarylamine) • MDOH • MDPR • MDPEA (Homopiperonylamine) • Mefenorex • Mephedrone • Mephentermine • Metanephrine • Metaraminol • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methoxamine • Methoxyphenamine • MMA • Methcathinone (Methylpropion) • Methedrone • Methoxyphenamine • Methylone • MMDA • MMDMA • MMMA • Morazone • N-Benzyl-1-phenethylamine • N,N-Dimethylphenethylamine • Naphthylamphetamine • Nisoxetine • Norepinephrine (Noradrenaline) • Norfenefrine • Norfenfluramine • Normetanephrine • Octopamine • Orciprenaline • Ortetamine • Oxilofrine • Paredrine (Norpholedrine, Oxamphetamine, Mycadrine) • PBA • PCA • PHA • Pargyline • Pentorex (Phenpentermine) • Pentylone • Phendimetrazine • Phenmetrazine • Phenpromethamine • Phentermine • Phenylalanine • Phenylephrine (Neosynephrine) • Phenylpropanolamine • Pholedrine • PIA • PMA • PMEA • PMMA • PPAP • Prenylamine • Propylamphetamine • Pseudoephedrine • Radafaxine • Ropinirole • Salbutamol (Albuterol; Levosalbutamol) • Sibutramine • Synephrine (Oxedrine) • Theodrenaline • Tiflorex (Flutiorex) • Tranylcypromine • Tyramine • Tyrosine • Xamoterol • Xylopropamine • Zylofuramine
Piperazines Piperidines 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine • 1-(3,4-Dichlorophenyl)-1-(piperidin-2-yl)butane • 2-Benzylpiperidine • 2-Methyl-3-phenylpiperidine • 3,4-Dichloromethylphenidate • 4-Benzylpiperidine • 4-Methylmethylphenidate • Desoxypipradrol • Difemetorex • Diphenylpyraline • Ethylphenidate • Methylnaphthidate • Methylphenidate (Dexmethylphenidate) • N-Methyl-3β-propyl-4β-(4-chlorophenyl)piperidine • Nocaine • Phacetoperane • Pipradrol • SCH-5472
Pyrrolidines 2-Diphenylmethylpyrrolidine • α-PPP • α-PBP • α-PVP • Diphenylprolinol • MDPPP • MDPBP • MDPV • MPBP • MPHP • MPPP • MOPPP • Naphyrone • PEP • Prolintane • Pyrovalerone
Tropanes 3-CPMT • 3'-Chloro-3α-(diphenylmethoxy)tropane • 3-Pseudotropyl-4-fluorobenzoate • 4'-Fluorococaine • AHN-1055 • Altropane (IACFT) • Brasofensine • CFT (WIN 35,428) • β-CIT (RTI-55) • Cocaethylene • Cocaine • Dichloropane (RTI-111) • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Norcocaine • PIT • PTT • RTI-31 • RTI-32 • RTI-51 • RTI-105 • RTI-112 • RTI-113 • RTI-117 • RTI-120 • RTI-121 (IPCIT) • RTI-126 • RTI-150 • RTI-154 • RTI-171 • RTI-177 • RTI-183 • RTI-193 • RTI-194 • RTI-199 • RTI-202 • RTI-204 • RTI-229 • RTI-241 • RTI-336 • RTI-354 • RTI-371 • RTI-386 • Salicylmethylecgonine • Tesofensine • Troparil (β-CPT, WIN 35,065-2) • Tropoxane • WF-23 • WF-33 • WF-60
Others 1-(Thiophen-2-yl)-2-aminopropane • 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-MDP • 2-Phenylcyclohexylamine • 2-Phenyl-3,6-dimethylmorpholine • 3-Benzhydrylmorpholine • 3,3-Diphenylcyclobutanamine • 5-(2-Aminopropyl)indole • 5-Iodo-2-aminoindane • AL-1095 • Amfonelic acid • Amineptine • Amiphenazole • Atipamezole • Atomoxetine (Tomoxetine) • Bemegride • Benzydamine • BTQ • BTS 74,398 • Carphedon • Ciclazindol • Cilobamine • Clofenciclan • Cropropamide • Crotetamide • Cypenamine • D-161 • Diclofensine • Dimethocaine • Efaroxan • Etamivan • EXP-561 • Fencamfamine • Fenpentadiol • Feprosidnine • G-130 • Gamfexine • Gilutensin • GSK1360707F • GYKI-52895 • Hexacyclonate • Idazoxan • Indanorex • Indatraline • JNJ-7925476 • JZ-IV-10 • Lazabemide • Leptacline • Levopropylhexedrine • Lomevactone • LR-5182 • Mazindol • Meclofenoxate • Medifoxamine • Mefexamide • Mesocarb • Methastyridone • Methiopropamine • N-Methyl-3-phenylnorbornan-2-amine • Nefopam • Nikethamide • Nomifensine • O-2172 • Oxaprotiline • Phthalimidopropiophenone • PNU-99,194 • Propylhexedrine • PRC200-SS • Rasagiline • Rauwolscine • Rubidium chloride • Setazindol • Tametraline • Tandamine • Trazium • UH-232 • Yohimbine
See also Sympathomimetic amines Dopaminergics Reuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugs Categories:- Alcohols
- Stimulants
- Norepinephrine-dopamine reuptake inhibitors
- Psychoactive drugs
- Pyrrolidines
- InChI=1S/C17H19NO/c19-17(16-12-7-13-18-16,14-8-3-1-4-9-14)15-10-5-2-6-11-15/h1-6,8-11,16,18-19H,7,12-13H2
Wikimedia Foundation. 2010.
