- 3-Methoxy-4-methylamphetamine
-
3-Methoxy-4-methylamphetamine 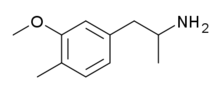
Systematic (IUPAC) name 1-(3-methoxy-4-methylphenyl)propan-2-amine Clinical data Pregnancy cat. ? Legal status Uncontrolled (but may be covered under the Federal Analogue Act in the United States and under similar bills in other countries) Routes Oral Identifiers CAS number 87179-33-7 ATC code None PubChem CID 91253 ChemSpider 82402 
ChEMBL CHEMBL47696 
Chemical data Formula C11H17NO Mol. mass 179.258 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)3-Methoxy-4-methylamphetamine (MMA) is an entactogen and psychedelic drug of the phenethylamine and amphetamine classes.[1][2] It was first synthesized in 1970[1] and was encountered as a street drug in Italy in the same decade.[3] MMA was largely forgotten until being reassayed by David E. Nichols as a non-neurotoxic MDMA analogue in 1991,[2] and has subsequently been sold as a designer drug on the internet since the late 2000s.[4]
In animal studies, MMA fully substitutes for MDMA and MBDB, partially substitutes for LSD, and does not substitute for amphetamine.[2] Additionally, it has been shown to potently inhibit the reuptake of serotonin and does not produce serotonergic neurotoxicity in rodents.[2] These data appear to confer a profile of MMA as a selective serotonin releasing agent (SSRA) and 5-HT2A receptor agonist.[2]
According to anecdotal reports, MMA is active in humans at doses of 20-60 mg and possesses a duration of approximately 12-16 hours.[4] It is said to produce empathy and mild 2C-like psychedelic effects, and although it is mood-lifting, it is not euphoric like MDMA and is more antidepressant-like in comparison.[4] At higher doses, such as 140 mg, the effects are much more psychedelic (comparable to DOM) but can be dysphoric and very long-lived.[5]
See also
- 3-Methoxyamphetamine (3-MeOA)
- 3-Methoxymethamphetamine (MMMA)
- 4-Methylamphetamine (4-MA)
- 4-Methylmethamphetamine (4-MMA)
- 4-Methylmethcathinone (4-MMC)
References
- ^ a b Ho BT, McIsaac WM, An R, et al. (January 1970). "Analogs of alpha-methylphenethylamine (amphetamine). I. Synthesis and pharmacological activity of some methoxy and/or methyl analogs". Journal of Medicinal Chemistry 13 (1): 26–30. doi:10.1021/jm00295a007. PMID 5412110.
- ^ a b c d e Johnson MP, Frescas SP, Oberlender R, Nichols DE (May 1991). "Synthesis and pharmacological examination of 1-(3-methoxy-4-methylphenyl)-2-aminopropane and 5-methoxy-6-methyl-2-aminoindan: similarities to 3,4-(methylenedioxy)methamphetamine (MDMA)". Journal of Medicinal Chemistry 34 (5): 1662–8. doi:10.1021/jm00109a020. PMID 1674539.
- ^ de Zorzi C, Cavalli A, Zacchia. 1974;10:3.
- ^ a b c "Bluelight Forums - MMA - 3-MeO-4-Me-Amphetamine". http://www.bluelight.ru/vb/showthread.php?t=362771.
- ^ Ann Shulgin; Alexander Shulgin (1991). Pihkal: A Chemical Love Story. Transform Press. ISBN 0-9630096-0-5. http://www.erowid.org/library/books_online/pihkal/pihkal123.shtml.
Entactogens Aminoindanes 5-IAI • ETAI • TAIAminotetralins 6-CATPhenethylamines
(and amphetamines,
cathinones, etc)4-CAB • 4-FA • 4-FMA • 4-MTA • 4-FPP • 5-APDB • 6-APDB • Ariadne • BDB • Brephedrone • Eutylone • Flephedrone • IAP • IMP • Metaescaline • Mephedrone • Methedrone • MMA • NAP • Norfenfluramine • Pentylone • PMA • PMEA • PMMA • TAPMDxx 2-Methyl-MDA • 5-Methyl-MDA • 6-Methyl-MDA • bk-MBDB (butylone) • bk-MDEA (ethylone) • bk-MDMA (methylone) • DMMDA • DMMDA-2 • EBDB • EBDP • EDMA • MDAI • MDMAI • MMAI • MDAT • MDMAT • MDA • MDDM • MDEA • MDIP • MDMA • MDMOH • MDMP • MDMPEA • MDOH • MDPEA • MDPH • MDPR • MMDPEA (Lophophine) • MBDB • MBDP • MMDA • MMDA-2 • MMDMATryptamines 4-Methyl-αET • αETSerotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507Reuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DCA • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersPhenethylamines Phenethylamines Psychedelics: 2C-B • 2C-B-FLY • 2C-C • 2C-D • 2C-E • 2C-F • 2C-G • 2C-I • 2C-N • 2C-P • 2C-SE • 2C-T • 2C-T-2 • 2C-T-4 • 2C-T-7 • 2C-T-8 • 2C-T-9 • 2C-T-13 • 2C-T-15 • 2C-T-17 • 2C-T-21 • 2C-TFM • 2C-YN • Allylescaline • DESOXY • Escaline • Isoproscaline • Jimscaline • Macromerine • MEPEA • Mescaline • Metaescaline • Methallylescaline • Proscaline • Psi-2C-T-4 • TCB-2
Stimulants: 2-OH-PEA • β-Me-PEA • Hordenine • N-Me-PEA • Phenethylamine (PEA)
Entactogens: Lophophine • MDPEA • MDMPEA
Others: BOH • DMPEAAmphetamines
PhenylisopropylaminesPsychedelics: 3C-BZ • 3C-E • 3C-P • Aleph • Beatrice • Bromo-DragonFLY • D-Deprenyl • DMA • DMCPA • DMMDA • DOB • DOC • DOEF • DOET • DOI • DOM • DON • DOPR • DOTFM • Ganesha • MMDA • MMDA-2 • Psi-DOM • TMA • TeMA
Stimulants: 4-MA • 4-MMA • 4-MTA • 5-IT • Alfetamine • Amfecloral • Amfepentorex • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Benfluorex • Benzphetamine • Cathine • Clobenzorex • Dimethylamphetamine • Ephedrine (EPH) • Ethylamphetamine • Fencamfamine • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenproporex • Fludorex • Furfenorex • Isopropylamphetamine • Lefetamine • Mefenorex • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methoxyphenamine • MMA • Norfenfluramine • Oxilofrine • Ortetamine • PBA • PCA • Phenpromethamine • PFA • PFMA • PIA • PMA • PMEA • PMMA • Phenylpropanolamine (PPA) • Prenylamine • Propylamphetamine • Pseudoephedrine (PSE) • Sibutramine • Tiflorex (Flutiorex) • Tranylcypromine • Xylopropamine • Zylofuramine
Entactogens: 5-APDB • 6-APB • 6-APDB • EDA • IAP • MDA • MDEA • MDHMA (FLEA) • MDMA ("Ecstasy") • MDOH • MMDMA • NAP • TAP
Others: Amiflamine • DFMDA • D-Deprenyl • L-Deprenyl (Selegiline)Phentermines Stimulants: Chlorphentermine • Cloforex • Clortermine • Etolorex • Mephentermine • Pentorex (Phenpentermine) • Phentermine
Entactogens: MDPH • MDMPHCathinones Stimulants: Amfepramone • Brephedrone • Buphedrone • Bupropion (Amfebutamone) • Cathinone (Propion) • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Ethcathinone (Ethylpropion) • Flephedrone • Methcathinone (Methylpropion) • Mephedrone • Methedrone
Entactogens: Ethylone • MethylonePhenylisobutylamines Phenylalkylpyrrolidines Stimulants: α-PBP • α-PPP • α-PVP • MDPBP • MDPPP • MDPV • MOPPP • MPBP • MPHP • MPPP • Naphyrone • PEP • Prolintane • PyrovaleroneCatecholamines
(and relatives..)6-FNE • 6-OHDA • α-Me-DA • α-Me-TRA • Adrenochrome • Ciladopa • D-DOPA (Dextrodopa) • Dopamine • Epinephrine (Adrenaline) • Epinine • Fenclonine • Ibopamine • L-DOPA (Levodopa) • L-DOPS (Droxidopa) • L-Phenylalanine • L-Tyrosine • meta-Octopamine • meta-Tyramine • Metanephrine • Metirosine • Methyldopa • Nordefrin (Levonordefrin) • Norepinephrine (Noradrenaline) • Normetanephrine • para-Octopamine • para-TyramineMiscellaneous Amidephrine • Arbutamine • Cafedrine • Denopamine • Dobutamine • Dopexamine • Etafedrine • Ethylnorepinephrine • Etilefrine • Famprofazone • Gepefrine • Isoprenaline (Isoproterenol) • Isoetarine • Metaraminol • Metaterol • Methoxamine • Norfenefrine • Orciprenaline • Phenylephrine (Neosynephrine) • Phenoxybenzamine • Prenalterol • Pronethalol • Propranolol • Salbutamol (Albuterol; Levosalbutamol) • Synephrine (Oxedrine) • Theodrenaline • XamoterolCategories:- Amphetamines
- Entactogens and Empathogens
- Phenol ethers
Wikimedia Foundation. 2010.
