- 25B-NBOMe
-
25B-NBOMe 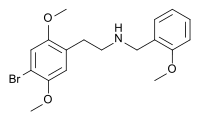
Systematic (IUPAC) name 2-(4-bromo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine Clinical data Pregnancy cat. ? Legal status ? Identifiers CAS number 1026511-90-9 ATC code ? PubChem CID 9977044 ChemSpider 8152636 
Chemical data Formula C18H22BrNO3 Mol. mass 380.275 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)25B-NBOMe (NBOMe-2C-B, BOM 2-CB, Cimbi-36) is a derivative of the phenethylamine hallucinogen 2C-B, discovered in 2004 by Ralf Heim at the Free University of Berlin. It acts as a potent partial agonist for the 5HT2A receptor.[1][2][3][4] Anecdotal reports from human users suggest 25B-NBOMe to be an active hallucinogen at a dose of as little as 400-650mcg insufflated, making it a similar potency to other phenethylamine derived hallucinogens such as bromo-dragonfly. Duration of effects lasts about 10h.
See also
- 2CBCB-NBOMe (NBOMe-TCB-2)
- 2CBFly-NBOMe (NBOMe-2CB-Fly)
- 2C-C-NBOMe (NBOMe-2CC)
- 25I-NBOMe (NBOMe-2CI)
- 2C-TFM-NBOMe (NBOMe-2C-TFM)
- 25I-NBMD (NBMD-2CI)
- 25I-NBOH (NBOH-2CI)
- 25I-NBF (NBF-2CI)
References
- ^ Ralf Heim PhD. Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Entwicklung eines neuen Struktur-Wirkungskonzepts. (German)
- ^ Maria Silva PhD. Theoretical study of the interaction of agonists with the 5-HT2A receptor. Universität Regensburg, 2009.
- ^ Ettrup, A.; Hansen, M.; Santini, M. A.; Paine, J.; Gillings, N.; Palner, M.; Lehel, S.; Herth, M. M. et al. (2010). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT2A agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging 38 (4): 681–693. doi:10.1007/s00259-010-1686-8. ISBN 0025901016868. PMID 21174090.
- ^ Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). "Theoretical studies on the interaction of partial agonists with the 5-HT(2A) receptor". Journal of Computer-aided Molecular Design 25 (1): 51–66. doi:10.1007/s10822-010-9400-2. PMID 21088982.
Categories:- Psychedelic phenethylamines
- Organobromides
- Phenol ethers
- Hallucinogen stubs
Wikimedia Foundation. 2010.
