- Bufotenin
-
Bufotenin 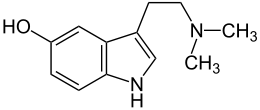
Systematic (IUPAC) name 3-(2-dimethylaminoethyl)-1H-indol-5-ol Clinical data Pregnancy cat. ? Legal status Schedule I (US) Routes Parenteral Identifiers CAS number 487-93-4 
ATC code None PubChem CID 10257 IUPHAR ligand 144 DrugBank DB01445 ChemSpider 9839 
KEGG C08299 
ChEBI CHEBI:3210 
ChEMBL CHEMBL416526 
Synonyms N,N-dimethyl-5-hydroxytryptamine, 5-hydroxy-dimethyltryptamine, bufotenine, cebilcin Chemical data Formula C12H16N2O Mol. mass 204.268 g/mol SMILES eMolecules & PubChem Physical data Melt. point 146–147 °C (295–297 °F) Boiling point 320 °C (608 °F)  (what is this?) (verify)
(what is this?) (verify)Bufotenin (also known as bufotenine and cebilcin), or 5-hydroxy-dimethyltryptamine (5-HO-DMT or 5-OH-DMT), is a tryptamine related to the neurotransmitter serotonin. It is an alkaloid found in the skin of some species of toads; in mushrooms, higher plants, and mammals.[1]
The name bufotenin originates from the Bufo genus of toads, which includes several species of psychoactive toads, most notably Bufo alvarius, that secrete bufotoxins from their parotoid glands.[2] Bufotenin is similar in chemical structure to the psychedelics psilocin (4-HO-DMT), 5-MeO-DMT, and DMT, chemicals which also occur in some of the same fungus, plant, and animal species as bufotenin. Psychedelic effects of bufotenin in humans have been observed in some studies.
Contents
Nomenclature
Bufotenin (bufotenine) is also known by the chemical names 5-hydroxy-dimethyltryptamine (5-HO-DMT), N,N-dimethyl-5-hydroxytryptamine, dimethyl serotonin,[3] and mappine.[3]
History
Bufotenin was first isolated, from toad skin, and named by the Austrian chemist Handovsky at the University of Prague during World War I.[4] The structure of bufotenine was first confirmed in 1934 by Heinrich Wieland’s laboratory in Munich, and the first reported synthesis of bufotenine was by Toshio Hoshino in 1936.[4]
Sources
Toads
See also: Psychoactive toadBufotenin is a chemical constituent in the venom and eggs of several species of toads belonging to the Bufo genus, but most notably in the Colorado River toad (Bufo alvarius). Extracts of toad venom, containing bufotenin and other bioactive compounds, have been used in some traditional medicines such as ch’an su (probably derived from Bufo gargarizans), which has been used medicinally for centuries in China.[5]
The toad was "recurrently depicted in Mesoamerican art,"[6] which some authors have interpreted as indicating that the effects of ingesting Bufo secretions have been known in Mesoamerica for many years; however, others doubt that this art provides sufficient "ethnohistorical evidence" to support the claim.[5]
In addition to bufotenine, Bufo venoms also contain digoxin-like cardiac glycosides, and ingestion of the venom can be fatal. Ingestion of Bufo toad venom and eggs by humans has resulted in several reported cases of poisoning,[7][8][9] some of which resulted in death.[9][10][11]
Contemporary reports indicate that bufotenine-containing toad venom has been used as a street drug; that is, as an aphrodisiac, ingested orally in the form of ch’an su,[9] and as a psychedelic, by smoking or orally ingesting Bufo toad venom or dried Bufo skins. The use of chan'su and love stone (a related toad venom preparation used as an aphrodisiac in the West Indies) has resulted in several cases of poisoning and at least one death.[9][12] The practice of orally ingesting toad venom has been referred to in popular culture and in the scientific literature as toad licking and has drawn media attention.[13][14] Albert Most, founder of the Church of the Toad of Light and a proponent of recreational use of Bufo alvarius venom, published a booklet titled Bufo alvarius: The Psychedelic Toad of the Sonoran Desert[15][16] in 1983 which explained how to extract and smoke the secretions.
Bufotenin is also present in the skin secretion of three arboreal amphibian species of the Osteocephalus genus (Osteocephalus taurinus, Osteocephalus oophagus, and Osteocephalus langsdorffii) from the Amazon and Atlantic rain forests.[17]
Anadenanthera seeds
Bufotenin is a constituent of the seeds of Anadenanthera colubrina and Anadenanthera peregrina trees. Anadenanthera seeds have been used as an ingredient in psychedelic snuff preparations by indigenous cultures of Central and South America.[18]
Mushrooms
Bufotenin is also allegedly found in several species of Amanita mushrooms, including Amanita muscaria, Amanita citrina, and Amanita porphyria, though this is widely disputed and likely untrue.[4]
Other sources
Bufotenin has been identified as a component in the latex of the takini (Brosimum acutifolium) tree, which is used as a psychedelic by South American shamans,[19] and in the seeds of Mucuna pruriens [20]
Pharmacology
Uptake and elimination
In rats, subcutaneously administered bufotenin (1–100 μg/kg) distributes mainly to the lungs, heart, and blood, and to a much lesser extent, the brain (hypothalamus, brain stem, striatum, and cerebral cortex) and liver. It reaches peak concentrations at 1 hour and is nearly completely eliminated within 8 hours.[21] In humans, intravenous administration of bufotenin results in excretion of (70%) of injected drug in the form of 5-HIAA, an endogenous metabolite of serotonin, while roughly 4% is eliminated unmetabolized in the urine. Orally administered bufotenine undergoes extensive first-pass metabolism by the enzyme monoamine oxidase.
Lethal dose
The acute toxicity (LD50) of bufotenin in rodents has been estimated at 200 to 300 mg/kg. Death occurs by respiratory arrest.[18]
Effects in humans
Fabing & Hawkins (1955)
In 1955, Fabing and Hawkins administered bufotenin intravenously at doses of up to 16 mg to prison inmates at Ohio State Penitentiary.[22] A troubling toxic blood circulation effect causing a purpling of the face was seen in these tests.
A subject given 1 mg reported “a tight feeling in the chest” and prickling “as if he had been jabbed by needles.” This was accompanied by a “fleeting sensation of pain in both thighs and a mild nausea.” [22]
Another subject given 2 mg reported “tightness in his throat”. He had tightness in the stomach, tingling in pretibial areas, and developed a purplish hue in the face indicating blood circulation problems. He vomited after 3 minutes.[22]
Another subject given 4 mg complained of “chest oppression” and that “a load is pressing down from above and my body feels heavy.” The subject also reported “numbness of the entire body” and “a pleasant Martini feeling-my body is taking charge of my mind”. The subject reported he saw red spots passing before his eyes and red-purple spots on the floor, and the floor seemed very close to his face. Within 2 minutes these visual effects were gone, and replaced by a yellow haze, as if he were looking through a lens filter.[22]
Fabing and Hawkins commented that bufotenin’s psychedelic effects were "reminiscent of LSD and mescaline but develop and disappear more quickly, indicating rapid central action and rapid degradation of the drug".
Isbell (1956)
In 1956, Dr. Harris S. Isbell at the Public Health Service Hospital in Lexington, Kentucky experimented with bufotenine as a snuff. He reported “no subjective or objective effects were observed after spraying with as much as 40 mg bufotenine”; however subjects who received 10–12 mg injected intramuscularly reported “elements of visual hallucinations consisting of a play of colors, lights, and patterns”.[4]
Turner & Merlis (1959)
Turner and Merlis (1959) [23] experimented with intravenous administration of bufotenine (as the water soluble creatinine sulfate salt) to schizophrenics at a New York state hospital. They reported that when one subject received 10 mg during a 50-second interval, “the peripheral nervous system effects were extreme: at 17 seconds, flushing of the face, at 22 seconds, maximal inhalation, followed by maximal hyperventilation for about 2 minutes, during which the patient was unresponsive to stimuli; her face was plum-colored". Finally, Turner and Merlis reported that:
- “on one occasion, which essentially terminated our study, a patient who received 40 mg intramuscularly, suddenly developed an extremely rapid heart rate; no pulse could be obtained; no blood pressure measured. There seemed to have been an onset of auricular fibrillation…extreme cyanosis developed. Massage over the heart was vigorously executed and the pulse returned to normal…shortly thereafter the patient, still cyanotic, sat up saying: ‘Take that away. I don’t like them’.”
After pushing doses to the morally admissible limit without producing visuals, Turner and Merlis conservatively concluded: “We must reject bufotenine…as capable of producing the acute phase of Cohoba intoxication”.[4]
McLeod and Sitaram (1985)
A 1985 study by McLeod and Sitaram in humans reported that bufotenine administered intranasally at a dose of 1–16 mg had no effect, other than intense local irritation. When given intravenously at low doses (2–4 mg), bufotenine oxalate caused anxiety but no other effects; however, a dose of 8 mg resulted in profound emotional and perceptual changes, involving extreme anxiety, a sense of imminent death, and visual disturbance associated with color reversal and distortion, and intense flushing of the cheeks and forehead.[24]
Ott (2001)
In 2001, ethnobotanist Jonathan Ott published the results of a study in which he self-administered free base bufotenine via insufflation (5–100 mg), sublingually (50 mg), intrarectally (30 mg), orally (100 mg) and via vaporization (2–8 mg).[25] Ott reported “visionary effects" of intranasal bufotenine and that the "visionary threshold dose" by this route was 40 mg, with smaller doses eliciting perceptibly psychoactive effects. He reported that "intranasal bufotenine is throughout quite physically relaxing; in no case was there facial rubescence, nor any discomfort nor disesteeming side effects".
At 100 mg, effects began within 5 minutes, peaked at 35–40 minutes, and lasted up to 90 minutes. Higher doses produced effects that were described as psychedelic, such as "swirling, colored patterns typical of tryptamines, tending toward the arabesque". Free base bufotenin taken sublingually was found to be identical to intranasal use. The potency, duration, and psychedelic action was the same. Ott found vaporized free base bufotenin active from 2–8 mg with 8 mg producing "ring-like, swirling, colored patterns with eyes closed". He noted that the visionary effects of insufflated bufotenine were verified by one colleague, and those of vaporized bufotenine by several volunteers.
Ott concluded that free base bufotenin taken intranasally and sublingually produced effects similar to those of Yopo without the toxic peripheral symptoms, such as facial flushing, observed in other studies in which the drug was administered intravenously.
Association with schizophrenia and other mental disorders
A study conducted in the late 1960s reported the detection of bufotenin in the urine of schizophrenic subjects;[26] however, subsequent research has failed to confirm these findings.[27][28][29][30]
Studies have detected endogenous bufotenin in urine specimens from individuals with other psychiatric disorders,[31] such as infant autistic patients.[32] Another study indicated that paranoid violent offenders or those who committed violent behaviour towards family members have higher bufotenin levels in their urine than other violent offenders.[33]
A 2010 study utilized a mass spectrometry approach to detect levels of bufotenin in the urine of individuals with severe autism spectrum disorder (ASD), schizophrenia, and asymptomatic subjects. Their results indicate significantly higher levels of bufotenin in the urine of the ASD and schizophrenic groups when compared to asymptomatic individuals.[34]
Legal status
Bufotenine is regulated as a Schedule I drug (ID number 7403) by the U.S. Drug Enforcement Agency.[3] It is classified as a Schedule I controlled substance according to the Criminal Code Regulations of the Government of the Commonwealth of Australia.[35]
In the UK, the substance is a Class A drug under the 1971 Misuse of Drugs Act, although numerous shops sell Amanita muscaria across the Internet. Although some people claim that they may contain the substance, it is often considered an area of dispute and based on unverified claims.
See also
- List of entheogens
- Tryptamines
- Psychoactive toad
- Bufo alvarius
- Anadenanthera colubrina
- Anadenanthera peregrina
References
- ^ CID 10257. PubChem. Accessed on May 6, 2007.
- ^ Bufo Alvarius. AmphibiaWeb. Accessed on May 6, 2007.
- ^ a b c "DEA Drug Scheduling". U.S. Drug Enforcement Agency. http://www.usdoj.gov/dea/pubs/scheduling.html. Retrieved 2007-08-11.
- ^ a b c d e Chilton WS, Bigwood J, Jensen RE (1979). "Psilocin, bufotenine and serotonin: historical and biosynthetic observations". J Psychedelic Drugs. 11 (1–2): 61–9. PMID 392119.
- ^ a b Davis W, Weil A (1992). "Identity of a New World Psychoactive Toad". Ancient Mesoamerica 3: 51–9.
- ^ Kennedy AB (1982). "Ecce Bufo: The Toad in Nature and in Olmec Iconography". Current Anthropology 23 (3): 273–90. doi:10.1086/202831.
- ^ Hitt M, Ettinger DD (1986). "Toad toxicity". N Engl J Med 314 (23): 1517–8. doi:10.1056/NEJM198606053142320. PMID 3702971.
- ^ Ragonesi DL (1990). "The boy who was all hopped up". Contemporary Pediatrics 7: 91–4.
- ^ a b c d Brubacher JR, Ravikumar PR, Bania T, Heller MB, Hoffman RS (1996). "Treatment of toad venom poisoning with digoxin-specific Fab fragments". Chest 110 (5): 1282–8. doi:10.1378/chest.110.5.1282. PMID 8915235.
- ^ Gowda RM, Cohen RA, Khan, IA (2003). "Toad venom poisoning: resemblance to digoxin toxicity and therapeutic implications". Heart 89 (4): e14. doi:10.1136/heart.89.4.e14. PMC 1769273. PMID 12639891. http://heart.bmj.com/cgi/content/abstract/89/4/e14.
- ^ Lever, Christopher (2001). The Cane Toad: The History and Ecology of a Successful Colonist. Westbury Academic & Scientific Publishing. ISBN 1-84103-006-6.
- ^ Centers for Disease Control and Prevention (CDC) (1995). "Deaths associated with a purported aphrodisiac—New York City, February 1993-May 1995". MMWR Morb Mortal Wkly Rep 44 (46): 853–5, 861. PMID 7476839. http://www.cdc.gov/mmwr/preview/mmwrhtml/00039633.htm.
- ^ The Dog Who Loved to Suck on Toads. NPR. Accessed on May 6, 2007.
- ^ Psychoactive toad: Cultural references
- ^ Most, A. "Bufo avlarius: The Psychedelic Toad of the Sonoran Desert". www.erowid.org Family Guy season 2 episode 14 Lets Go to the Hop.. http://www.erowid.org/archive/sonoran_desert_toad/almost.htm. Retrieved 2007-08-12.
- ^ How ‘bout them toad suckers? Ain’t they clods? Smoky Mountain News. Accessed on May 6, 2007
- ^ Costa TO, Morales RA, Brito JP, Gordo M, Pinto AC, Bloch C Jr. (2005). "Occurrence of bufotenin in the Osteocephalus genus (Anura: Hylidae)". Toxicon 46 (4): 371–5. doi:10.1016/j.toxicon.2005.02.006. PMID 16054186.
- ^ a b Repke, David B.; Torres, Constantino Manuel (2006). Anadenanthera: visionary plant of ancient South America. New York: Haworth Herbal Press. ISBN 0-7890-2642-2.
- ^ Moretti C, Gaillard Y, Grenand P, Bévalot F, Prévosto JM (2006). "Identification of 5-hydroxy-tryptamine (bufotenine) in takini (Brosimumacutifolium Huber subsp. acutifolium C.C. Berg, Moraceae), a shamanic potion used in the Guiana Plateau". J Ethnopharmacol 106 (2): 198–202. doi:10.1016/j.jep.2005.12.022. PMID 16455218.
- ^ Chamakura RP (1994). "Bufotenine—a hallucinogen in ancient snuff powders of South America and a drug of abuse on the streets of New York City". Forensic Sci Rev 6 (1): 2–18.
- ^ Fuller RW, Snoddy HD, Perry KW (1995). "Psilocin Tissue distribution, metabolism and effects of bufotenine administered to rats". Neuropharmacology 34 (7): 799–804. doi:10.1016/0028-3908(95)00049-C. PMID 8532147.
- ^ a b c d Fabing HD, Hawkins, JR (1956). "Intravenous bufotenine injection in the human being". Science 123 (3203): 886–7. doi:10.1126/science.123.3203.886. PMID 13324106.
- ^ Turner WJ, Merlis S (1959). "Effects of some indolealkylamines on man". Arch Neurol Psychiatr 81: 121–9.
- ^ McLeod WR, Sitaram BR (1985). "Bufotenine reconsidered". Acta Psychiatrica Scandinavica 72 (5): 447–50. doi:10.1111/j.1600-0447.1985.tb02638.x.
- ^ Ott J (2001). "Pharmanopo-psychonautics: human intranasal, sublingual, intrarectal, pulmonary and oral pharmacology of bufotenine". J Psychoactive Drugs 33 (4): 403–7. PMID 11824699. http://entheology.org/edoto/anmviewer.asp?a=9&z=8.
- ^ [Faurbye A, Pind K (November 1968). "Occurrence of bufotenin in the urine of schizophrenic patients and normal persons". Nature 220 (5166): 489. doi:10.1038/220489a0. PMID 5686166. http://www.nature.com/nature/journal/v220/n5166/abs/220489a0.html.
- ^ Siegel M (October 1965). "A sensitive method for the detection of n,n-dimethylserotonin (bufotenin) in urine; failure to demonstrate its presence in the urine of schizophrenic and normal subjects". J Psychiatr Res 3 (3): 205–11. doi:10.1016/0022-3956(65)90030-0. PMID 5860629.
- ^ Pomilio AB, Vitale AA, Ciprian-Ollivier J, Cetkovich-Bakmas M, Gómez R, Vázquez G. (1999). "Ayahoasca: an experimental psychosis that mirrors the transmethylation hypothesis of schizophrenia". J Ethnopharmacol 65 (1): 29–51. doi:10.1016/S0378-8741(98)00163-9. PMID 10350367.
- ^ Ciprian-Ollivier J, Cetkovich-Bakmas MG (1997). "Altered consciousness states and endogenous psychoses: a common molecular pathway?". Schizophrenia Research 28 (2–3): 257–65. doi:10.1016/S0920-9964(97)00116-3. PMID 9468359.
- ^ Carpenter WT Jr, Fink EB, Narasimhachari N, Himwich HE (1975). "A test of the transmethylation hypothesis in acute schizophrenic patients". Am J Psychiatry 132 (10): 1067–71. PMID 1058643.
- ^ Takeda N, Ikeda R, Ohba K, Kondo M (November 1995). "Bufotenine reconsidered as a diagnostic indicator of psychiatric disorders". Neuroreport 6 (17): 2378–80. doi:10.1097/00001756-199511270-00024. PMID 8747157.
- ^ Takeda N (February 1994). "Serotonin-degradative pathways in the toad (Bufo bufo japonicus) brain: clues to the pharmacological analysis of human psychiatric disorders". Comp Biochem Physiol Pharmacol Toxicol Endocrinol 107 (2): 275–81. doi:10.1016/1367-8280(94)90051-5. PMID 7749594.
- ^ Räisänen MJ, Virkkunen M, Huttunen MO, Furman B, Kärkkäinen J (September 1984). "Increased urinary excretion of bufotenin by violent offenders with paranoid symptoms and family violence". Lancet 2 (8404): 700–1. doi:10.1016/S0140-6736(84)91263-7. PMID 6147728.
- ^ Emanuele E, Colombo R, Martinelli V, Brondino N, Marini M, Boso M, Barale F, Politi P (2010). "Elevated urine levels of bufotenine in patients with autistic spectrum disorders and schizophrenia". Neuro Endocrinol Lett 31 (1): 117–21. PMID 20150873.
- ^ (rtf) Criminal Code Regulation 2005 (SL2005-2). Australian Capital Territory. May 1, 2005. http://www.legislation.act.gov.au/sl/2005-2/20050501-18968/rtf/2005-2.rtf. Retrieved 2007-08-12.
External links
Drugs from TiHKAL AL-LAD • DBT • DET • DiPT • 5-MeO-α-MT • DMT • 2,α-DMT • α,N-DMT • DPT • EiPT • α-ET • ETH-LAD • Harmaline • Harmine • 4-HO-DBT • 4-HO-DET • 4-HO-DiPT • 4-HO-DMT • 5-HO-DMT • 4-HO-DPT • 4-HO-MET • 4-HO-MiPT • 4-HO-MPT • 4-HO-pyr-T • Ibogaine • LSD • MBT • 4,5-MDO-DiPT • 5,6-MDO-DiPT • 4,5-MDO-DMT • 5,6-MDO-DMT • 5,6-MDO-MiPT • 2-Me-DET • 2-Me-DMT • Melatonin • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 4-MeO-MiPT • 5-MeO-MiPT • 5,6-MeO-MiPT • 5-MeO-NMT • 5-MeO-pyr-T • 6-MeO-THH • 5-MeO-TMT • 5-MeS-DMT • MiPT • α-MT • NET • NMT • PRO-LAD • pyr-T • Tryptamine • Tetrahydroharmine • α,N,O-TMS
Serotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969 • Vortioxetine
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507 • VortioxetineReuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DCA • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersTryptamines 1-(2-Dimethylaminoethyl)dihydropyrano(3,2-e)indole • 2-Methyl-5-HT • 4-Acetoxy-DET • 4-Acetoxy-DIPT • 4-Acetoxy-DMT • 4-HO-αMT • 4-HO-DIPT • 4-HO-MET • 4-MeO-DMT • 4-Methyl-αET • 4-Methyl-αMT • 5-Benzyloxytryptamine • 5-Bromo-DMT • 5-Carboxamidotryptamine • 5-Ethoxy-αMT • 5-Fluoro-αMT • 5-HO-αMT • 5-HTP • 5-Ethoxy-DMT • 5-Ethyl-DMT • 5-Fluoro-DMT • 5-Methyl-DMT • 5-Methoxytryptamine • 5-MeO-7,N,N-TMT • 5-Methyl-αET • 5-MeO-αET • 5-MeO-αMT • 5-MeO-DALT • 5-MeO-DET • 5-MeO-DIPT • 5-MeO-DMT • 5-MeO-DPT • 5-MeO-MALT • 5-MeO-MIPT • 5,7-Dihydroxytryptamine • 5-(Nonyloxy)tryptamine • 6-Fluoro-αMT • 7-Methyl-αET • 7-Methyl-DMT • αET • αMT • Aeruginascin • AL-37350A • BW-723C86 • Baeocystin • Bufotenidine • Bufotenin • DALT • Desformylflustrabromine • DET • DiPT • DMT • DPT • Ethocybin • EiPT • EMDT • EPT • Ethocin • FGIN-127 • FGIN-143 • Ibogaine • Indorenate • Iprocin • MET • MiPT • Miprocin • Melatonin • MS-245 • NAS • NMT • Norbaeocystin • Normelatonin • Oxypertine • PiPT • Psilocin • Psilocybin • Rizatriptan • Serotonin • Sumatriptan • Tryptamine • Tryptophan • Yohimbine • Yuremamine • Zolmitriptan
Neurotransmitters Amino acids Alanine · Aspartate · Cycloserine · DMG · GABA · Glutamate · Glycine · Hypotaurine · Kynurenic acid (Transtorine) · NAAG (Spaglumic acid) · NMG (Sarcosine) · Serine · Taurine · TMG (Betaine)
Endocannabinoids 2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide · PEA (Palmitoylethanolamide) · RVD-Hpα · Hp (Hemopressin)
Gasotransmitters Monoamines Purines Trace amines 3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
Others 1,4-BD · Acetylcholine · GBL · GHB · Histamine
See also Template:NeuropeptidesCategories:- Entheogens
- Psychedelic tryptamines
- Natural tryptamine alkaloids
- Vertebrate toxins
- Phenols
Wikimedia Foundation. 2010.
