- Oxalate
-
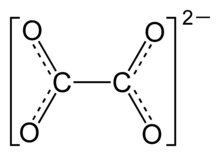
Oxalate (IUPAC: ethanedioate), is the dianion with formula C2O42− also written (COO)22−. Either name is often used for derivatives, such as disodium oxalate, (Na+)2C2O42−, or an ester of oxalic acid (such as dimethyl oxalate, (CH3)2C2O4. Oxalate also forms coordination compounds where it is sometimes abbreviated as ox.
Many metal ions form insoluble precipitates with oxalate, a prominent example being calcium oxalate, the primary constituent of the most common kind of kidney stones.
Contents
Relationship to oxalic acid
The dissociation of protons from oxalic acid proceeds in a stepwise manner as for other polyprotic acids. Loss of a single proton results in the monovalent hydrogenoxalate anion HC2O4−. A salt with this anion is sometimes called an acid oxalate, monobasic oxalate, or hydrogen oxalate. The equilibrium constant (Ka) for loss of the first proton is 5.37×10-2 (pKa = 1.27). The loss of the second proton, which yields the oxolate ion has a equilibrium constant of 5.25×10-5 (pKa = 4.28). These values imply that, in solutions with neutral pH, there is no oxalic acid, and only trace amounts of hydrogen oxalate.[1] The literature is often unclear on the distinction between H2C2O4, HC2O4-, and C2O42-, and the collection of species is referred to oxalic acid.
Occurrence in nature
Oxalate occurs in many plants, where it is synthesized via the incomplete oxidation of carbohydrates.
Oxalate-rich plants include fat hen ("lamb's quarters"), sorrel, and several Oxalis species. The root and/or leaves of rhubarb and buckwheat are high in oxalic acid.[2] Other edible plants that contain significant concentrations of oxalate include—in decreasing order—star fruit (carambola), black pepper, parsley, poppy seed, amaranth, spinach, chard, beets, cocoa, chocolate, most nuts, most berries, fishtail palms, New Zealand spinach (Tetragonia tetragonioides) and beans.[citation needed] Leaves of the tea plant (Camellia sinensis) contain among the greatest measured concentrations of oxalic acid relative to other plants. However the infusion beverage typically contains only low to moderate amounts of oxalic acid per serving, due to the small mass of leaves used for brewing.
Common high-oxalate foods[3] Food Item Serving
(oz.)Oxalate
Content
(mg)Beet greens, cooked 1/2 cup 916 Purslane, leaves, cooked 1/2 cup 910 Rhubarb, stewed, no sugar 1/2 cup 860 Spinach, cooked 1/2 cup 750 Beets, cooked 1/2 cup 675 Chard, Swiss, leaves cooked 1/2 cup 660 Rhubarb, canned 1/2 cup 600 Spinach, frozen 1/2 cup 600 Beets, pickled 1/2 cup 500 Poke Greens, cooked 1/2 cup 476 Endive, raw 20 long leaves 273 Cocoa, dry 1/3 cup 254 Dandelion greens, cooked 1/2 cup 246 Okra, cooked 8 - 9 pods 146 Potatoes, sweet, cooked 1/2 cup 141 Kale, cooked 1/2 cup 125 Peanuts, raw 1/3 cup (1-3/4 oz) 113 Turnip greens, cooked 1/2 cup 110 Chocolate, unsweetened 1 oz. 91 Parsnips, diced, cooked 1/2 cup 81 Collard greens, cooked 1/2 cup 74 Pecans, halves, raw 1/3 cup (1-1/4 oz) 74 Tea, leaves (4 min. infusion) 1 level tsp in 7 oz water 72 Cereal germ, toasted 1/4 cup 67 Gooseberries 1/2 cup 66 Potato, Idaho white, baked 1 medium 64 Carrots, cooked 1/2 cup 45 Apple, raw with skin 1 medium 41 Brussel sprouts, cooked 6 - 8 medium 37 Strawberries, raw 1/2 cup 35 Celery, raw 2 stalks 34 Milk chocolate bar 1 bar (1.02 oz) 34 Raspberries, black, raw 1/2 cup 33 Orange, edible portion 1 medium 24 Green beans, cooked 1/2 cup 23 Chives, raw, chopped 1 tablespoon 19 Leeks, raw 1/2 medium 15 Blackberries, raw 1/2 cup 13 Concord grapes 1/2 cup 13 Blueberries, raw 1/2 cup 11 Currants, red 1/2 cup 11 Apricots, raw 2 medium 10 Raspberries, red, raw 1/2 cup 10 Broccoli, cooked 1 large stalk 6 Cranberry juice 1/2 cup (4 oz) 6 The "gritty mouth" feeling one experiences when drinking milk with a rhubarb dessert is caused by precipitation of calcium, abstracted from the casein in dairy products, as calcium oxalate.[citation needed]
Physiological effects
In the body, oxalic acid combines with divalent metallic cations such as calcium (Ca2+) and iron(II) (Fe2+) to form crystals of the corresponding oxalates which are then excreted in urine as minute crystals. These oxalates can form larger kidney stones that can obstruct the kidney tubules. An estimated 80% of kidney stones are formed from calcium oxalate.[4] Those with kidney disorders, gout, rheumatoid arthritis, or certain forms of chronic vulvar pain (vulvodynia) are typically advised to avoid foods high in oxalic acid. Methods to reduce the oxalate content in food are of current interest.[5]
Magnesium (Mg2+) oxalate is 567 times more soluble than calcium oxalate, so the latter is more likely to precipitate out when magnesium levels are low and calcium and oxalate levels are high.
Magnesium oxalate is a million times more soluble than mercury oxalate. Oxalate solubility for other metals decreases in the order Ca > Cd > Zn > {Mn,Ni,Fe,Cu} > {As,Sb,Pb} > Hg. The highly insoluble iron(II) oxalate appears to play a major role in gout, in the nucleation and growth of the otherwise extremely soluble sodium urate. This explains why gout usually appears after age 40, when ferritin levels in blood exceed 100 ng/dl. Beer is rich in oxalate and iron, and ethanol increases iron absorption and magnesium elimination, so beer intake greatly increases the risk of a gout attack.
Cadmium catalyzes the transformation of vitamin C into oxalic acid. This can be a problem for people exposed to high levels of cadmium in the diet, in the workplace, or through smoking.
In studies with rats, calcium supplements given along with foods high in oxalic acid can cause calcium oxalate to precipitate out in the gut and reduce the levels of oxalate absorbed by the body (by 97% in some cases.)[6][7]
Oxalic acid can also be produced by the metabolism of ethylene glycol ("antifreeze"), glyoxylic acid, or ascorbic acid (vitamin C).[8][dubious ]
Powdered oxalate is used as a pesticide in beekeeping to combat the bee mite.
Some fungi of the genus Aspergillus produce oxalic acid, which reacts with calcium from the blood or tissue to precipitate calcium oxalate.[9]
There is some preliminary evidence that the administration of probiotics can affect oxalic acid excretion rates[10] (and presumably oxalic acid levels as well.)
As a ligand
Oxalate, the conjugate base of oxalic acid, is an excellent ligand for metal ions. It usually binds as a bidentate ligand forming a 5-membered MO2C2 ring. An illustrative complex is potassium ferrioxalate, K3[Fe(C2O4)3]. The drug Oxaliplatin exhibits improved water solubility relative to older platinum-based drugs, avoiding the dose-limiting side-effect of nephrotoxicity. Oxalic acid and oxalates can be oxidized by permanganate in an autocatalytic reaction. One of the main applications of oxalic acid is a rust-removal, which arises because oxalate forms water soluble derivatives with the ferric ion.
Safety
Although unusual, consumption of oxalates (for example, the grazing of animals on oxalate-containing plants such as greasewood or human consumption of Sorrel) may result in kidney disease or even death due to oxalate poisoning. The presence of Oxalobacter formigenes in the gut flora can prevent this. Cadmium catalyzes the transformation of vitamin C into oxalic acid and can result from smoking heavily, ingesting produce tainted with Cd or from industrial exposure to Cd.
See also
Raphides
Oxalate salts
- sodium oxalate - Na2C2O4
- calcium oxalate - CaC2O4, a major component of kidney stones
Oxalate complexes
- potassium ferrioxalate - K3[Fe(C2O4)3], an iron complex with oxalate ligands
Oxalate esters
- diphenyl oxalate - (C6H5)2C2O4
- dimethyl oxalate - (CH3)2C2O4
References
- ^ Wilhelm Riemenschneider, Minoru Tanifuji "Oxalic Acid" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a18_247.
- ^ Streitweiser, Andrew Jr.; Heathcock, Clayton H.: Introduction to Organic Chemistry, Macmillan 1976, p 737
- ^ Resnick, Martin I.; Pak, Charles Y. C. (1990). Urolithiasis, A Medical and Surgical Reference. W.B. Saunders Company. pp. 158. ISBN 0721624391.
- ^ Coe FL, Evan A, Worcester E. (2005). "Kidney stone disease". J Clin Invest. 115 (10): 2598–608. doi:10.1172/JCI26662. PMC 1236703. PMID 16200192. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1236703.
- ^ Betsche, T.; Fretzdorff, B. (2005). "Biodegradation of oxalic acid from spinach using cereal radicles". J Agric Food Chem. 53 (25): 9751–8. doi:10.1021/jf051091s. PMID 16332126.
- ^ Morozumi M, Hossain RZ, Yamakawa KI, Hokama S, Nishijima S, Oshiro Y, Uchida A, Sugaya K, Ogawa Y (2006). "Gastrointestinal oxalic acid absorption in calcium-treated rats". Urol Res 34 (3): 168. doi:10.1007/s00240-006-0035-7. PMID 16444511.
- ^ Hossain RZ, Ogawa Y, Morozumi M, Hokama S, Sugaya K (2003). "Milk and calcium prevent gastrointestinal absorption and urinary excretion of oxalate in rats". Front Biosci. 8 (1-3): a117–25. doi:10.2741/1083. PMID 12700095.
- ^ Mandl J, Szarka A, Bánhegyi G (2009). "Vitamin C: update on physiology and pharmacology". British Journal of Pharmacology 157 (7): 1097–1110. doi:10.1111/j.1476-5381.2009.00282.x. PMC 2743829. PMID 19508394. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2743829.
- ^ Pabuccuoglu U. (2005). "Aspects of oxalosis associated with aspergillosis in pathology specimens". Pathol Res Pract. 201 (5): 363–8. doi:10.1016/j.prp.2005.03.005. PMID 16047945.
- ^ Lieske JC, Goldfarb DS, De Simone C, Regnier C. (2005). "Use of a probiotic to decrease enteric hyperoxaluria". Kidney Int. 68 (3): 1244–9. doi:10.1111/j.1523-1755.2005.00520.x. PMID 16105057.
Categories:- Oxalates
- Carboxylate anions
Wikimedia Foundation. 2010.