- Hirudin
-
Hirudin 
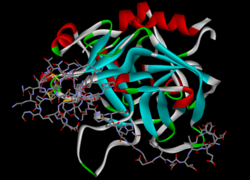
crystallographic analysis at 3.0-angstroms resolution of the binding to human thrombin of four active site-directed inhibitors Identifiers Symbol Hirudin Pfam PF00713 InterPro IPR000429 SCOP 4htc Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Hirudin is a naturally occurring peptide in the salivary glands of medicinal leeches (such as Hirudo medicinalis) that has a blood anticoagulant property. This is fundamental for the leeches’ alimentary habit of hematophagy, since it keeps the blood flowing after the initial phlebotomy performed by the worm on the host’s skin.
Contents
Structure
During his years in Birmingham and Edinburgh, John Berry Haycraft had been actively engaged in research and published papers on the coagulation of blood and in 1884, he discovered that the leech secreted a powerful anticoagulant, which he named hirudin, although it was not isolated until the 1950s, nor its structure fully determined until 1976. Full length, hirudin is made up of 65 amino acids. These amino acids are organised into a compact N-terminal domain containing three disulfide bonds and a C-terminal domain, which is completely disordered, when the protein is un-complexed in solution.[2][3] Amino acid residues 1-3 form a parallel beta- strand with residues 214-217 of thrombin, the nitrogen atom of residue 1 making a hydrogen bond with the Ser-195 O gamma atom of the catalytic site. The C-terminal domain makes numerous electrostatic interactions with an anion-binding exosite of thrombin, while the last five residues are in a helical loop that forms many hydrophobic contacts.[4] Natural hirudin contains a mixture of various isoforms of the protein. However, recombinant techniques can be used to produce homogeneous preparations of hirudin.[5]
Biological activity
A key event in the final stages of blood coagulation is the conversion of fibrinogen into fibrin by the serine protease enzyme thrombin.[6] Thrombin is produced from prothrombin, by the action of an enzyme, prothrombinase, in the final states of coagulation. Fibrin is then cross linked by factor XIII to form a blood clot. The principal inhibitor of thrombin in normal blood circulation is antithrombin III.[5] Similar to antithrombin III, the anticoagulatant activity of hirudin is based on its ability to inhibit the pro-coagulant activity of thrombin.
Hirudin is the most potent natural inhibitor of thrombin. Unlike antithrombin III, hirudin binds to and inhibits only the activity of thrombin, with a specific activity on fibrinogen.[5] Therefore, hirudin prevents or dissolves the formation of clots and thrombi (i.e., it has a thrombolytic activity), and has therapeutic value in blood coagulation disorders, in the treatment of skin hematomas and of superficial varicose veins, either as an injectable or a topical application cream. In some aspects, hirudin has advantages over more commonly used anticoagulants and thrombolytics, such as heparin, as it does not interfere with the biological activity of other serum proteins and can also act on complexed thrombin.
It is difficult to extract large amounts of hirudin from natural sources, so a method for producing and purifying this protein using recombinant biotechnology has been developed. This has led to the development and marketing of a number of hirudin-based anticoagulant pharmaceutical products such as Lepirudin (Refludan) , Hirudin derived from Hansenula (Thrombexx, Extrauma) and Desirudin (Revasc/Iprivask). Several other direct thrombin inhibitors are derived chemically from hirudin.
See also
- Hirudotherapy
References
- ^ PDB 4HTC
- ^ Folkers PJM, Clore GM. et al. (1989). "Solution structure of recombinant hirudin and the Lys-47-Glu mutant: a nuclear magnetic resonance and hybrid distance geometry-dynamical simulated annealing study". Biochemistry 28 (6): 2601–2617. doi:10.1021/bi00432a038. PMID 2567183.
- ^ Haruyama H. and Wuthrich K. (1989). "Conformation of recombinant desulfatohirudin in aqueous solution determined by nuclear magnetic resonance". Biochemistry 28 (10): 4301–4312. doi:10.1021/bi00436a027. PMID 2765488.
- ^ Rydel TJ, Ravichandran KG, Tulinsky A, Bode W, Huber R, Roitsch C, Fenton JW (July 1990). "The structure of a complex of recombinant hirudin and human alpha-thrombin". Science 249 (4966): 277-80. PMID 2374926.
- ^ a b c Rydell TJ, Tulinsky A. et al. (1991). "Refined structure of the Hirudin-Thrombin complex". J. Mol. Biol. 221 (2): 583–601. doi:10.1016/0022-2836(91)80074-5. PMID 1920434.
- ^ Fenton JW 2nd, Ofosu SA et al. (1998). "Thrombin and antithrombotics". Semin Thromb Hemost 24 (2): 87–91. doi:10.1055/s-2007-995828. PMID 9579630.
External links
Categories:- Direct thrombin inhibitors
- Peptides
- Medicine stubs
Wikimedia Foundation. 2010.