- Clopidogrel
-
Clopidogrel 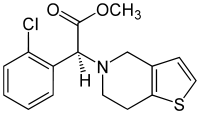
Systematic (IUPAC) name (+)-(S)-methyl 2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)acetate Clinical data Trade names Plavix AHFS/Drugs.com monograph MedlinePlus a601040 Pregnancy cat. B1(AU) B(US) Legal status Prescription Only (S4) (AU) POM (UK) ℞-only (US) Routes Oral Pharmacokinetic data Bioavailability >50% Protein binding 94–98% Metabolism Hepatic Half-life 7–8 hours (inactive metabolite) Excretion 50% renal
46% biliaryIdentifiers CAS number 113665-84-2 ATC code B01AC04 PubChem CID 60606 DrugBank APRD00444 ChemSpider 54632 
UNII A74586SNO7 
KEGG D07729 
ChEBI CHEBI:37941 
ChEMBL CHEMBL1771 
Chemical data Formula C16H16ClNO2S Mol. mass 321.82 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Clopidogrel is an oral, thienopyridine class antiplatelet agent used to inhibit blood clots in coronary artery disease, peripheral vascular disease, and cerebrovascular disease. It is marketed by Bristol-Myers Squibb and Sanofi-Aventis under the trade name Plavix. The drug works by irreversibly inhibiting a receptor called P2Y12, an adenosine diphosphate ADP chemoreceptor. Adverse effects include hemorrhage, severe neutropenia, and thrombotic thrombocytopenic purpura (TTP).
Contents
Pharmacology
Clopidogrel is a prodrug, the action of which may be related to an adenosine diphosphate (ADP) receptor on platelet cell membranes. The drug specifically and irreversibly inhibits the P2Y12 subtype of ADP receptor, which is important in aggregation of platelets and cross-linking by the protein fibrin.[1] The blockade of this receptor inhibits platelet aggregation by blocking activation of the glycoprotein IIb/IIIa pathway. The IIb/IIIa complex functions as a receptor mainly for fibrinogen and vitronectin but also for fibronectin and von Willebrand factor. Activation of this receptor complex is the "final common pathway" for platelet aggregation and is important in the cross-linking of platelets by fibrin.
Platelet inhibition can be demonstrated two hours after a single dose of oral clopidogrel, but the onset of action is slow, so that a loading-dose of 300–600 mg is usually administered.
Chemistry
Due to opening of the thiophene ring, the metabolite chemical structure now has three sites of chirality, making a total of eight possible isomers. These are; a stereocentre at C4 (attached to the —SH thiol group), a stereobond at C3—C16 double bound and the original stereocentre at C7. Only one of the eight structures is an active antiplatelet drug. This has the following configuration; a (Z) configuration at C3—C16 double bound, the original (S) configuration stereocentre at C7 and although the stereocentre at C4 can't be directly determined, as the thiol group is too reactive, work with the active metabolite of the related drug Prasugrel suggests that the (R)-configuration of the C4 group is critical for P2Y12 and platelet-inhibitory activities. [2]
Clinical use
Indications
Clopidogrel is indicated for:[3]
- Prevention of vascular ischemic events in patients with symptomatic atherosclerosis
- Acute coronary syndrome without ST-segment elevation (NSTEMI),
- ST elevation MI (STEMI)
It is also used, along with aspirin, for the prevention of thrombosis after placement of intracoronary stent[3] or as an alternative antiplatelet drug for patients who are intolerant to aspirin.[4]
International guidelines granted the highest grade of recommendation for NSTE-ACS, PCI and stent,[clarification needed] for clopidogrel in addition to aspirin. Consensus-based therapeutic guidelines recommend also the use of clopidogrel, instead of aspirin, in patients requiring antiplatelet therapy but with a history of gastric ulceration, as inhibition of the synthesis of prostaglandins by aspirin (acetylsalicylic acid) can exacerbate this condition. A study has shown that in patients with healed aspirin-induced ulcers, however, patients receiving aspirin plus the proton pump inhibitor esomeprazole had a lower incidence of recurrent ulcer bleeding than patients receiving clopidogrel.[5] However, a more recent study suggested that prophylaxis with proton pump inhibitors along with clopidogrel following acute coronary syndrome may increase adverse cardiac outcomes, possibly due to inhibition of CYP2C19, which is required for the conversion of clopidogrel to its active form.[6][7][8] The European Medicines Agency has issued a public statement on a possible interaction between clopidogrel and proton pump inhibitors.[9] However, several cardiologists have voiced concern that the studies on which these warnings are based have many limitations and that it is not certain whether there really is an interaction between clopidogrel and proton pump inhibitors.[10]
Dosage forms
Clopidogrel is marketed as clopidogrel bisulfate (clopidogrel hydrogen sulfate), most commonly under the trade names Plavix, as 75 mg oral tablets.
Pharmacokinetics and metabolism
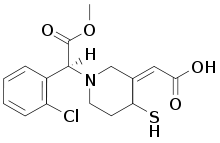 The active metabolite of clopidogrel [11]
The active metabolite of clopidogrel [11]
After repeated 75-mg oral doses of clopidogrel (base), plasma concentrations of the parent compound, which has no platelet inhibiting effect, are very low and are generally below the quantification limit (0.000258 mg/L) beyond two hours after dosing.
Clopidogrel is a pro-drug activated in the liver by cytochrome P450 enzymes, including CYP2C19. The active metabolite has an elimination half-life of about eight hours and acts by forming a disulfide bridge with the platelet ADP receptor. Patients with a variant allele of CYP2C19 are 1.5 to 3.5 times more likely to die or have complications than patients with the high-functioning allele.[12][13][14]
Following an oral dose of 14C-labeled clopidogrel in humans, approximately 50% was excreted in the urine and approximately 46% in the feces in the five days after dosing.
Effect of Food: Administration of clopidogrel bisulfate with meals did not significantly modify the bioavailability of clopidogrel as assessed by the pharmacokinetics of the main circulating metabolite.
Absorption and Distribution: Clopidogrel is rapidly absorbed after oral administration of repeated doses of 75 mg clopidogrel (base), with peak plasma levels (appx. 3 mg/L) of the main circulating metabolite occurring approximately one hour after dosing. The pharmacokinetics of the main circulating metabolite are linear (plasma concentrations increased in proportion to dose) in the dose range of 50 to 150 mg of clopidogrel. Absorption is at least 50% based on urinary excretion of clopidogrel-related metabolites. Clopidogrel and the main circulating metabolite bind reversibly in vitro to human plasma proteins (98% and 94%, respectively). The binding is nonsaturable in vitro up to a concentration of 110 μg/mL.
Metabolism and Elimination: In vitro and in vivo, clopidogrel undergoes rapid hydrolysis into its carboxylic acid derivative. In plasma and urine, the glucuronide of the carboxylic acid derivative is also observed.
In March 2010, the U.S. Food and Drug Administration (FDA) added a boxed warning to Plavix alerting that the drug can be less effective in people who cannot metabolize the drug to convert it to its active form.[15][16]
Pharmacogenetics
CYP2C19 is an important drug-metabolizing enzyme that catalyzes the biotransformation of many clinically useful drugs including antidepressants, barbiturates, proton pump inhibitors, antimalarial and antitumor drugs. Clopidogrel is one of the drugs metabolized by this enzyme.
Several recent landmark studies have proven the importance of 2C19 genotyping in treatment using clopidogrel or Plavix. In March 2010, the FDA put a black box warning on Plavix to make patients and healthcare providers aware that CYP2C19 poor metabolizers, representing up to 14% of patients, are at high risk of treatment failure and that testing is available.[17] Researchers have found that patients with variants in cytochrome P-450 2C19 (CYP2C19) have lower levels of the active metabolite of clopidogrel, less inhibition of platelets, and a 3.58 times greater risk for major adverse cardiovascular events such as death, heart attack, and stroke; the risk was greatest in CYP2C19 poor metabolizers.[18]
Adverse effects
Serious adverse drug reactions associated with clopidogrel therapy include:
- Severe neutropenia (low white blood cells) (Incidence: 1/2,000)
- Thrombotic thrombocytopenic purpura (TTP) (Incidence: 4/1,000,000 patients treated)[19][20]
- Hemorrhage - The annual incidence of hemorrhage may be increased by the co-administration of aspirin.[21]
- Gastrointestinal Hemorrhage (Incidence: 2.0% annually)
- Cerebral Hemorrhage (Incidence: 0.1 to 0.4% annually)
- Use of non-steroidal anti-inflammatory drugs is discouraged in those taking clopidogrel due to increased risk of digestive tract hemorrhage
- Certain serious side effects include: skin irritations, respiratory difficulties, bloody stools and vomit, bloating of facial features, limbs and joints, exhaustion etc.
Interactions
Clopidogrel interacts with the following drugs: proton pump inhibitors (except possibly pantoprazole), phenytoin (Dilantin); tamoxifen (Nolvadex); tolbutamide (Orinase); torsemide (Demadex); fluvastatin (Lescol); a blood thinner such as warfarin (Coumadin), heparin, ardeparin (Normiflo), dalteparin (Fragmin), danaparoid (Orgaran), enoxaparin (Lovenox), or tinzaparin (Innohep); (Activase), anistreplase (Eminase), dipyridamole (Persantine), streptokinase (Kabikinase, Streptase), ticlopidine (Ticlid), and urokinase (Abbokinase).
In November 2009, the FDA announced that clopidogrel should not be taken with proton pump inhibitors such as omeprazole and esomeprazole.[22][23]
Marketing and litigation
Plavix is marketed worldwide in nearly 110 countries, with sales of US$6.6 billion in 2009.[24] It had been the 2nd top selling drug in the world for a few years as of 2007[25] and was still growing by over 20% in 2007. U.S. sales were US$3.8 billion in 2008.[26]
In 2006, generic clopidogrel was briefly marketed by Apotex, a Canadian generic pharmaceutical company before a court order halted further production until resolution of a patent infringement case brought by Bristol-Myers Squibb.[27] The court ruled that Bristol-Myers Squibb's patent was valid and provided protection until November 2011.[28]
Generic clopidogrel is also produced by several pharmaceutical companies in India. Clopidogrel is marketed by Sun Pharmaceuticals under the trade name Clopilet, by Ranbaxy Laboratories under the trade name Ceruvin, and under the name "Clavix" by Intas Pharmaceuticals and under the name "Deplatt" by Torrent Pharmaceuticals. In India, it is sold as Clopigrel, Clopitab, Clopijoy, and Clasprin (mixed with aspirin).
References
- ^ Savi, P; Zachayus JL, Delesque-Touchard N et al. (July 2006). "The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts". Proceedings of the National Academy of Sciences of the USA 103 (29): 11069–11074. doi:10.1073/pnas.0510446103. PMC 1635153. PMID 16835302. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1635153.
- ^ Pereillo JM, Maftouh M, Andrieu A, Uzabiaga MF, Fedeli O, Savi P, Pascal M, Herbert JM, Maffrand JP, Picard C (2002). "Structure and stereochemistry of the active metabolite of clopidogrel". Drug Metab. Dispos. 30 (11): 1288–95. doi:10.1124/dmd.30.11.1288. PMID 12386137. http://dmd.aspetjournals.org/cgi/reprint/30/11/1288.pdf.
- ^ a b Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- ^ Michael D Randall; Karen E Neil (2004). Disease management. 2nd ed. London: Pharmaceutical Press. 159.
- ^ Chan FK, Ching JY, Hung LC, et al. (2005). "Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding". N. Engl. J. Med. 352 (3): 238–44. doi:10.1056/NEJMoa042087. PMID 15659723.
- ^ Mistry SD, Trivedi HR, Parmar DM, Dalvi PS, Jiyo C. (2011). "Impact of proton pump inhibitors on efficacy of clopidogrel: Review of evidence". Indian Journal of Pharmacology 43 (2): 183–6. doi:10.4103/0253-7613.77360. PMC 3081459. PMID 21572655. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3081459.
- ^ Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, Rumsfeld JS. (2009). "Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome". Journal of the American Medical Association 301 (9): 937–44. doi:10.1001/jama.2009.261. PMID 19258584.
- ^ Stockl, KM; et al. (2010). "Risk of Rehospitalization for Patients Using Clopidogrel With a Proton Pump Inhibitor". Arch Intern Med (American Medical Association) 170 (8): 704–710. doi:10.1001/archinternmed.2010.34. ISSN 1538-3679. PMID 20421557. http://archinte.ama-assn.org/cgi/reprint/170/8/704.
- ^ Wathion, Noël. "Public statement on possible interaction between clopidogrel and proton pump inhibitors". http://www.ema.europa.eu. http://www.ema.europa.eu. http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2009/11/WC500014409.pdf. Retrieved 31 March 2011.
- ^ Hughes, Sue. "EMEA issues warning on possible clopidogrel-PPI interaction, but is there really a problem?". http://www.theheart.org. http://www.theheart.org/article/980779.do. Retrieved 31 March 2011.
- ^ Pereillo JM, Maftouh M, Andrieu A, Uzabiaga MF, Fedeli O, Savi P, Pascal M, Herbert JM, Maffrand JP, Picard C (2002). "Structure and stereochemistry of the active metabolite of clopidogrel". Drug Metab. Dispos. 30 (11): 1288–95. doi:10.1124/dmd.30.11.1288. PMID 12386137. http://dmd.aspetjournals.org/cgi/reprint/30/11/1288.pdf.
- ^ Mega JL; Close, SL; Wiviott, SD; Shen, L; Hockett, RD; Brandt, JT; Walker, JR; Antman, EM et al. (January 2009). "Cytochrome p-450 polymorphisms and response to clopidogrel". The New England Journal of Medicine 360 (4): 354–62. doi:10.1056/NEJMoa0809171. PMID 19106084.
- ^ Simon T; Verstuyft, C; Mary-Krause, M; Quteineh, L; Drouet, E; Méneveau, N; Steg, PG; Ferrières, J et al. (January 2009). "Genetic Determinants of Response to Clopidogrel and Cardiovascular Events". The New England Journal of Medicine 360 (4): 363–75. doi:10.1056/NEJMoa0808227. PMID 19106083.
- ^ Collet, J; Hulot, JS; Pena, A; Villard, E; Esteve, JB; Silvain, J; Payot, L; Brugier, D et al. (January 2009). "Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study". The Lancet 373 (9660): 309–17. doi:10.1016/S0140-6736(08)61845-0. PMID 19108880.
- ^ "FDA Announces New Boxed Warning on Plavix: Alerts patients, health care professionals to potential for reduced effectiveness" (Press release). Food and Drug Administration (United States). March 12, 2010. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm204253.htm. Retrieved March 13, 2010.
- ^ "FDA Drug Safety Communication: Reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug". Drug Safety and Availability. Food and Drug Administration (United States). March 12, 2010. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm. Retrieved March 13, 2010.
- ^ http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm204253.htm
- ^ PGxNews.Org (June 2009). "FDA updates Plavix label with PGx data". PGxNews.Org. http://www.pgxnews.org/web/the-news/1-latest-news/47-fda-updates-plavix-label-with-pgx-data. Retrieved 2009-06-13.[dead link]
- ^ Zakarija A, Bandarenko N, Pandey DK, et al. (2004). "Clopidogrel-Associated TTP An Update of Pharmacovigilance Efforts Conducted by Independent Researchers, Pharmaceutical Suppliers, and the Food and Drug Administration". Stroke 35 (2): 533–8. doi:10.1161/01.STR.0000109253.66918.5E. PMID 14707231.
- ^ {Clopidogrel (Plavix) [package insert]. New York,NY: Bristol-Myers Squibb and Sanofi-Synthelabo; May 2002.}
- ^ Diener HC, Bogousslavsky J, Brass LM, et al. (2004). "Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial". Lancet 364 (9431): 331–7. doi:10.1016/S0140-6736(04)16721-4. PMID 15276392.
- ^ DeNoon, Daniel J. "FDA Warns Plavix Patients of Drug Interactions", WebMD, 2009-11-19. Retrieved on 2009-11-23.
- ^ "Public Health Advisory: Updated Safety Information about a drug interaction between Clopidogrel Bisulfate (marketed as Plavix) and Omeprazole (marketed as Prilosec and Prilosec OTC)". Drug Safety and Availability. Food and Drug Administration (United States). November 17, 2009. http://web.archive.org/web/20091229162205/http://www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm190825.htm. Retrieved March 13, 2010.
- ^ "New products and markets fuel growth in 2005". IMS Health. http://www1.imshealth.com/web/content/0,3148,64576068_63872702_70260998_77974518,00.html. Retrieved 2009-03-02.
- ^ "Top Ten Global Products - 2007" (PDF). IMS Health. 2008-02-26. http://www.imshealth.com/deployedfiles/imshealth/Global/Content/StaticFile/Top_Line_Data/Top10GlobalProducts.pdf. Retrieved 2009-03-02.
- ^ "Details for Plavix". http://drugpatentwatch.com/ultimate/preview/tradename/index.php?query=PLAVIX.
- ^ "Preliminary Injunction Against Apotex Upheld on Appeal" (Press release). Bristol-Myers Squibb. December 8, 2006. http://www.businesswire.com/news/bms/20081215006516/en/Preliminary-Injunction-Apotex-Upheld-Appeal. Retrieved 2010-03-14.
- ^ "U.S. judge upholds Bristol, Sanofi patent on Plavix". Reuters. June 19, 2007. http://www.reuters.com/article/2007/06/19/us-bristolmyers-plavix-patent-idUSN1931607820070619. Retrieved 2007-09-05.
External links
- 3418 drug label/data at Daily Med from U.S. National Library of Medicine, National Institutes of Health.
- Plavix official website Bristol-Myers Squibb/Sanofi-Aventis
- Plavix prescribing information Bristol-Myers Squibb/Sanofi-Aventis
- Plavix, Aspirin and Stents : Patients' Forum : Angioplasty.Org
- Drug promises end to migraine misery
- U.S. National Library of Medicine: Drug Information Portal - Clopidogrel
Categories:- ADP receptor inhibitors
- Sanofi
- Bristol-Myers Squibb
- Prodrugs
- Thienopyridines
- Organochlorides
- Methyl esters
- Carboxylate esters
Wikimedia Foundation. 2010.

