- Cilostazol
-
Cilostazol 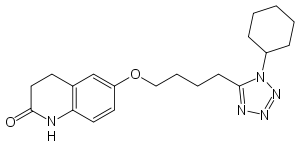
Systematic (IUPAC) name 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-
3,4-dihydro-2(1H)-quinolinoneClinical data Trade names Pletal AHFS/Drugs.com monograph MedlinePlus a601038 Pregnancy cat. C(US) Legal status ? Routes Oral Pharmacokinetic data Protein binding 95–98% Metabolism Hepatic (CYP3A4- and CYP2C19-mediated) Half-life 11–13 hours Excretion Renal Identifiers CAS number 73963-72-1 ATC code B01AC23 PubChem CID 2754 DrugBank APRD00155 ChemSpider 2652 
UNII N7Z035406B 
KEGG D01896 
ChEBI CHEBI:31401 
ChEMBL CHEMBL799 
Chemical data Formula C20H27N5O2 Mol. mass 369.46 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Cilostazol (
 /sɨˈlɒstəzɒl/) is a medication used in the alleviation of the symptom of intermittent claudication in individuals with peripheral vascular disease. It is manufactured by Otsuka Pharmaceutical Co. under the trade name Pletal.
/sɨˈlɒstəzɒl/) is a medication used in the alleviation of the symptom of intermittent claudication in individuals with peripheral vascular disease. It is manufactured by Otsuka Pharmaceutical Co. under the trade name Pletal.Although drugs similar to cilostazol have increased the risk of death in patients with congestive heart failure, studies of significant size have not addressed people without the disease.
Cilostazol is a phosphodiesterase inhibitor with therapeutic focus on cAMP. It inhibits platelet aggregation and is a direct arterial vasodilator. Its main effects are dilation of the arteries supplying blood to the legs and decreasing platelet coagulation.
Contents
Mechanism
Cilostazol is a selective inhibitor of 3-type phosphodiesterase (PDE3) with therapeutic focus on increasing cAMP. An increase in cAMP results in an increase in the active form of PKA, which is directly related with an inhibition in platelet aggregation.
Clinical use
Cilostazol is approved for the treatment of intermittent claudication. The typical dose is 100 mg twice a day. The effects may take as long as 3 months to be evident and has been shown to improve pain-free walking distance by 50%.
In people with heart failure
Cilostazol, clearly effective for a debilitating condition whose current treatment is often inadequate, is a member of a pharmacologic class that is dangerous to people with severe heart failure and unstudied in other people. Cilostazol has been studied in people without heart failure, without evidence of harm, but much more data would be needed to determine that there is no risk at all. Although cilostazol would not be approvable for a trivial condition the Cardio-Renal Advisory Committee and FDA concluded that fully informed patients and physicians should be able to choose to use it to treat intermittent claudication. Patient and physician labeling will describe the basis for concern and the incomplete information available.[1]
Adverse effects
Possible side effects of cilostazol use include headache (the most common), diarrhea, abnormal stools, increased heart rate, and palpitations.[2]
Interactions
Cilostazol is metabolized by CYP3A4 and CYP2C19, two isoenzymes of the cytochrome P450 system. Drugs that inhibit CYP3A4, such as itraconazole, erythromycin, ketoconazole, and diltiazem, are known to interact with cilostazol. The proton pump inhibitor omeprazole, a potent inhibitor of CYP2C19, increases exposure to the active metabolite of cilostazol.[2]
There has been a single report of grapefruit juice possibly increasing the effects of cilostazol;[3] some drug information sources list this as a possible interaction.[4][5][6] The FDA-approved labeling of cilostazol notes that grapefruit juice (which is a CYP3A4 inhibitor) increases the drug's maximum concentration by around 50%.[2]
References
- ^ Center for Drug Evaluation and Research (August 11, 1999). "Approval of Cilostazol". U.S. Food and Drug Administration. http://www.fda.gov/cder/news/cilostazol/approval.htm. Retrieved 2007-04-30.
- ^ a b c "Cilostazol: Official FDA information, side effects and uses.". Drugs.com. February 2008. http://www.drugs.com/pro/cilostazol.html. Retrieved 2008-09-22.
- ^ Taniguchi K, Ohtani H, Ikemoto T, Miki A, Hori S, Sawada Y (October 2007). "Possible case of potentiation of the antiplatelet effect of cilostazol by grapefruit juice". J Clin Pharm Ther 32 (5): 457–9. doi:10.1111/j.1365-2710.2007.00844.x. PMID 17875111.
- ^ "Cilostazol for peripheral arterial disease". Yahoo! Health. http://health.yahoo.com/other-other/cilostazol-for-peripheral-arterial-disease/healthwise--aa127481.html. Retrieved 2008-09-21.
- ^ "Cilostazol". MedicineNet.com. May 25, 1999. http://www.medicinenet.com/cilostazol/article.htm. Retrieved 2008-09-22.
- ^ Cerner-Multum, Inc. (November 29, 2007). "Consumer Drug Information: Cilostazol". Drugs.com. http://www.drugs.com/mtm/cilostazol.html. Retrieved 2008-09-22.
External links
- Supplementary information provided by Drug Digest
- U.S. National Library of Medicine: Drug Information Portal - Cilostazol
Categories:- Antiplatelet drugs
- Vasodilators
- Tetrazoles
- Quinolines
- Lactams
- Phenol ethers
- PDE3 inhibitors
Wikimedia Foundation. 2010.
