- Protein kinase A
-
In cell biology, Protein kinase A (PKA) refers to a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase (EC 2.7.11.11). Protein kinase A has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism.
It should neither be confused with AMP-activated protein kinase - which, although being of similar nature, may have opposite effects - [1] nor with cyclin-dependent kinases (Cdks).
Contents
Mechanism
Activation
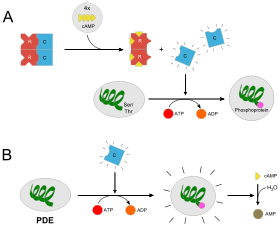
Each PKA is a holoenzyme that consists of two regulatory and two catalytic subunits. Under low levels of cAMP, the holoenzyme remains intact and is catalytically inactive. When the concentration of cAMP rises (e.g., activation of adenylate cyclases by G protein-coupled receptors coupled to Gs, inhibition of phosphodiesterases that degrade cAMP), cAMP binds to the two binding sites on the regulatory subunits, which leads to the release of the catalytic subunits.
Catalysis
The free catalytic subunits can then catalyse the transfer of ATP terminal phosphates to protein substrates at serine, or threonine residues. This phosphorylation usually results in a change in activity of the substrate. Since PKAs are present in a variety of cells and act on different substrates, PKA and cAMP regulation are involved in many different pathways.
The mechanisms of further effects may be divided into direct protein phosphorylation and protein synthesis:
- In direct protein phosphorylation, PKA directly either increases or decreases the activity of a protein.
- In protein synthesis, PKA first directly activates CREB, which binds the cAMP response element, altering the transcription and therefore the synthesis of the protein. In general, this mechanism takes more time (hours to days).
Inactivation
Thus, PKA is controlled by cAMP. Also, the catalytic subunit itself can be down-regulated by phosphorylation.
Downregulation of protein kinase A occurs by a feedback mechanism: One of the substrates that are activated by the kinase is a phosphodiesterase, which quickly converts cAMP to AMP, thus reducing the amount of cAMP that can activate protein kinase A.
Anchorage
The 2 regulatory subunits of protein kinase A are important for localizing the kinase inside the cell. With the aid of A-kinase anchor protein (AKAP), AKAP binds both to the regulatory subunits and to either a component of cytoskeleton structure or a membrane of an organelle, anchoring the enzyme complex to a particular subcellular compartment.
The catalytic function of protein kinase A would sometimes couple with the AKAP, binding PKA with phosphodiesterase to form a complex that functions as a signal module. For example, an AKAP locating near the nucleus of a heart muscle cell would bind to both PKA and phosphodiesterase that hydrolyzes cAMP. As phosphodiesterase contributes to the steady low concentration of cAMP in unstimulated cells, as the cell is stimulated, PKA is then responsible for the activation of phosphodiesterase (adjacent to PKA) in order to lower the concentration of cAMP. In this condition, as PKA and phosphodiesterase have formed a complex, the proximity increases the efficiency of PKA's activity.
Function
PKA phosphorylates other proteins, altering their function. As protein expression varies from cell type to cell type, the proteins that are available for phosphorylation will depend upon the cell in which PKA is present. Thus, the effects of PKA activation vary with cell type:
Overview table
Cell type Organ/system Stimulators
ligands --> Gs-GPCRs
or PDE inhibitorsInhibitors
ligands --> Gi-GPCRs
or PDE stimulatorsEffects adipocyte - epinephrine --> β-adrenergic receptor
- glucagon --> Glucagon receptor
myocyte (skeletal muscle) muscular system - epinephrine --> β-adrenergic receptor
- produce glucose
- stimulate glycogenolysis
- phosphorylate glycogen phosphorylase via phosphorylase kinase (activating it)[2]
- phosphorylate Acetyl-CoA carboxylase (inhibiting it)
- inhibit glycogenesis
- phosphorylate glycogen synthase (inhibiting it)[2]
- stimulate glycolysis
- phosphorylate phosphofructokinase 2 (stimulating it, cardiomyocytes only)
- stimulate glycogenolysis
myocyte (cardiac muscle) muscular system - norepinephrine --> β-adrenergic receptor
- sequester Ca2+ in sarcoplasmic reticulum
- phosphorylates phospholamban[3]
hepatocyte liver - epinephrine --> β-adrenergic receptor
- glucagon --> Glucagon receptor
- produce glucose
- stimulate glycogenolysis
- phosphorylate glycogen phosphorylase (activating it)[2]
- phosphorylate Acetyl-CoA carboxylase (inhibiting it)
- inhibit glycogenesis
- phosphorylate glycogen synthase (inhibiting it)[2]
- stimulate gluconeogenesis
- phosphorylate fructose 2,6-bisphosphatase (stimulating it)
- inhibit glycolysis
- phosphorylate phosphofructokinase -2 phosphatase (activating it)
- phosphorylate pyruvate kinase (inhibiting it)
- stimulate glycogenolysis
neurons in nucleus accumbens nervous system dopamine --> dopamine receptor Activate reward system principal cells in kidney kidney - exocytosis of aquaporin 2 to apical membrane. [4]
- synthesis of aquaporin 2 [4]
- phosphorylation of aquaporin 2 (stimulating it)[4]
myocyte (smooth muscle) muscular system - β2 adrenergic agonists --> β-2 adrenergic receptor
- histamine --> Histamine H2 receptor
- prostacyclin --> prostacyclin receptor
- Prostaglandin D2 --> PGD2 receptor
- Prostaglandin E2 --> PGE2 receptor
- VIP --> VIP receptor
- L-Arginine --> imidazoline and α-2 receptor? (Gi-coupled)
- muscarinic agonists, e.g. acetylcholine --> muscarinic receptor M2
- NPY --> NPY receptor
Vasodilation Thick ascending limb cell kidney Vasopressin --> V2 receptor stimulate Na-K-2Cl symporter (perhaps only minor effect) [4] Cortical collecting tubule cell kidney Vasopressin --> V2 receptor stimulate Epithelial sodium channel (perhaps only minor effect) [4] Inner medullary collecting duct cell kidney Vasopressin --> V2 receptor - stimulate urea transporter 1
- urea transporter 1 exocytosis[5]
proximal convoluted tubule cell kidney PTH --> PTH receptor 1 Inhibit NHE3 --> ↓H+ secretion[6] juxtaglomerular cell kidney - adrenergic agonists --> β-receptor[7]
- agonists --> A2 receptor[7]
- dopamine --> dopamine receptor[7]
- glucagon --> glucagon receptor[7]
renin secretion In adipocytes and hepatocytes
Adrenaline and glucagon affect the activity of protein kinase A by changing the levels of cAMP in a cell via the G-protein mechanism, using adenylate cyclase. Protein Kinase A acts to phosphorylate many enzymes important in metabolism. For example, protein kinase A phosphorylates acetyl-CoA carboxylase and pyruvate dehydrogenase. Such covalent modification has an inhibitory effect on these enzymes, thus inhibiting lipogenesis and promoting net gluconeogenesis. Insulin, on the other hand, decreases the level of phosphorylation of these enzymes, which instead promotes lipogenesis. Recall that gluconeogenesis does not occur in myocytes.
In nucleus accumbens neurons
PKA helps transfer/translate the dopamine signal into cells. It has been found (postmortem) to be elevated in the brains of smokers, in the nucleus accumbens, which mediates reward and motivation: a part of the brain acted on by "virtually all" recreational drugs, as well as "in the area of the midbrain that responds to dopamine, which acts as a 'reward chemical' in smokers and former-smokers." [8]
See also
- Protein kinase
- Signal transduction
- G protein-coupled receptor
- Serine/threonine-specific protein kinase
- Myosin light-chain kinase
- cAMP-dependent pathway
References
- ^ Hallows KR, Alzamora R, Li H, et al. (April 2009). "AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells". Am. J. Physiol., Cell Physiol. 296 (4): C672–81. doi:10.1152/ajpcell.00004.2009. PMC 2670645. PMID 19211918. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2670645.
- ^ a b c d e Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4. Page 172
- ^ Rodriguez P, Kranias EG. (December 2005). ""Phospholamban: a key determinant of cardiac function and dysfunction."". Arch Mal Coeur Vaiss 98 (12): 1239–43. PMID 16435604.
- ^ a b c d e Walter F., PhD. Boron. Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. ISBN 1-4160-2328-3. Page 842
- ^ Walter F., PhD. Boron. Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. ISBN 1-4160-2328-3. Page 844
- ^ Walter F., PhD. Boron. Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. ISBN 1-4160-2328-3. Page 852
- ^ a b c d Walter F., PhD. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. pp. 1300. ISBN 1-4160-2328-3. Page 867
- ^ "Smoking alters brain 'like drugs'". BBC News. 2007-02-24. http://news.bbc.co.uk/2/hi/health/6378179.stm. Retrieved 2010-05-22.
External links
- MeSH Cyclic+AMP-Dependent+Protein+Kinases
- Drosophila cAMP-dependent protein kinase 1 - The Interactive Fly
MAP see MAP kinase pathwayCalcium Intracellular calcium-sensing proteins • Calcineurin • Calcium-calmodulin-dependent protein kinaseG protein cAMP: Heterotrimeric G protein (Gs/Gi) • Adenylate cyclase • cAMP • 3',5'-cyclic-AMP phosphodiesterase • Protein kinase A
cGMP: Guanylate cyclase • cGMP • 3',5'-cyclic-GMP phosphodiesterase • Protein kinase G
Beta-gamma complex Gβ (GNB1, GNB2, GNB3, GNB4, GNB5) • Gγ (GNGT1, GNGT2, GNG2, GNG3, GNG4, GNG5, GNG7, GNG8, GNG10, GNG11, GNG12, GNG13, BSCL2)
G protein-coupled receptor kinase • AMP-activated protein kinaseCyclin Lipid Phosphoinositide phospholipase C • Phospholipase c gammaOther protein kinase Serine/threonine: Casein kinase (1, 2) • eIF-2 kinase (EIF2AK3) • Glycogen synthase kinase (GSK1, GSK2, GSK-3, GSK3A, GSK3B) • IκB kinase (CHUK, IKK2, IKBKG) • Interleukin-1 receptor-associated kinase (IRAK1, IRAK2, IRAK3, IRAK4) • Lim kinase (LIMK1, LIMK2) • p21 activated kinases (PAK1, PAK2, PAK3, PAK4) • Rho-associated protein kinase (ROCK1, ROCK2) • Ribosomal s6 kinase (RPS6KA1)
Tyrosine: ZAP70 • Focal adhesion protein-tyrosine kinase (PTK2, PTK2B) • BTK
both: Dual-specificity kinaseOther phosphoprotein phosphatase Serine/threonine: Protein phosphatase 2
Tyrosine: protein tyrosine phosphatase: Receptor-like protein tyrosine phosphatase • Sh2 domain-containing protein tyrosine phosphatase
both: Dual-specificity phosphataseApoptosis see apoptosis signaling pathwayGTP-binding protein regulators Other Activating transcription factor 6 • Signal transducing adaptor protein • I-kappa B protein • Mucin-4 • Olfactory marker protein • Phosphatidylethanolamine binding protein • EDARADD • PRKCSHCategories:- Cell signaling
- Signal transduction
- Protein kinases
- EC 2.7.11
Wikimedia Foundation. 2010.