- Exocytosis
-
For the meaning of "exocytosis" in dermatopathology, see exocytosis (dermatopathology).
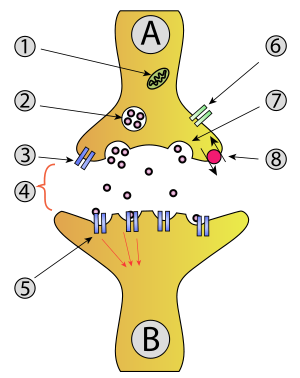 Neuron A (transmitting) to neuron B (receiving)
Neuron A (transmitting) to neuron B (receiving)
1. Mitochondrion
2. Synaptic vesicle with neurotransmitters
3. Autoreceptor
4. Synapse with neurotransmitter released (serotonin)
5. Postsynaptic receptors activated by neurotransmitter (induction of a postsynaptic potential)
6. Calcium channel
7. Exocytosis of a vesicle
8. Recaptured neurotransmitterExocytosis (/ˌɛksoʊsaɪˈtoʊsɪs/; from Greek ἔξω "out" and English cyto- "cell" from Gk. κύτος "receptacle"), also known as 'The peni-cytosis', is the durable process by which a cell directs the contents of secretory vesicles out of the cell membrane. These membrane-bound vesicles contain soluble proteins to be secreted to the extracellular environment, as well as membrane proteins and lipids that are sent to become components of the cell membrane.
Contents
Types
In multicellular organisms there are two types of exocytosis: 1) Ca2+ triggered non-constitutive and 2) non Ca2+ triggered constitutive. Exocytosis in neuronal chemical synapses is Ca2+ triggered and serves interneuronal signalling. Constitutive exocytosis is performed by all cells and serves the release of components of the extracellular matrix, or just delivery of newly-synthesized membrane proteins that are incorporated in the plasma membrane after the fusion of the transport vesicle. Regulated exocytosis, on the other hand, requires an external signal, a specific sorting signal on the vesicles, a clathrin coat, as well as an increase in intracellular calcium. Exocytosis is the opposite of endocytosis.
Steps
Five steps are involved in exocytosis:
Vesicle trafficking
Certain vesicle-trafficking steps require the translation of a vesicle over a moderately small distance. For example, vesicles that have the duty to transport the proteins from the Golgi apparatus to the cell surface area, will be likely to use motor proteins and a cytoskeletal track to get closer than previously stated to their target. Before tethering would have been appropriate, many of the proteins used for the active transport would have been instead set for passive transport, due to the fact that the Golgi apparatus does not require ATP to transport proteins. Both the actin- and the microtubule-base are implicated in these processes, along with several motor proteins. Once the vesicles reach their targets, they come into contact with tethering factors that can restrain them.
Vesicle tethering
It is useful to distinguish between the initial, loose tethering of vesicles with their objective from the more stable, packing interactions. Tethering involves links over distances of more than about half the diameter of a vesicle from a given membrane surface (>25 nm). Tethering interactions are likely to be involved in concentrating synaptic vesicles at the synapse.
The vesicles are also involved in regular cell's transcription processes.
Vesicle docking
The term docking refers to the holding of two membranes within a bilayer's distance of one another (<5-10 nm). Stable docking probably represents several distinct, molecular states: the molecular interactions underlying the close and tight association of a vesicle with its target may include the molecular rearrangements needed to trigger bilayer fusion. A common feature of many proteins that function in vesicle tethering and docking is their propensity to form highly extended, coiled-coil structures. Tethering and docking of a transport vesicle at the target membrane precedes the formation of a tight core SNARE complex.
Vesicle priming
In neuronal exocytosis, the term priming has been used to include all of the molecular rearrangements and ATP-dependent protein and lipid modifications that take place after initial docking of a synaptic vesicle but before exocytosis, such that the influx of calcium ions is all that is needed to trigger nearly instantaneous neurotransmitter release. In other cell types, whose secretion is constitutive (i.e. continuous, calcium ion independent, non-triggered) there is no priming.
Vesicle fusion
Further information: Vesicle fusionThe vesicle fusion is driven by SNARE proteins process of merging the vesicle membrane with the target one resulting in release of large biomolecules in the extracellular space (or in case of neurons in the synaptic cleft).
The merging of the donor and the acceptor membranes accomplishes three tasks:
- The surface of the plasma membrane increases (by the surface of the fused vesicle). This is important for the regulation of cell size, e.g., during cell growth.
- The substances within the vesicle are released into the exterior. These might be waste products or toxins, or signaling molecules like hormones or neurotransmitters during synaptic transmission.
- Proteins embedded in the vesicle membrane are now part of the plasma membrane. The side of the protein that was facing the inside of the vesicle now faces the outside of the cell. This mechanism is important for the regulation of transmembrane and transporters.
External links
See also
- Secretory pathway
- Synapse
- Endocytosis
- Phagocytosis
- Endocytic cycle
- Membrane nanotube
- Active Transport
- Viral shedding
- Presynaptic active zone
Membranes: Membrane transport Passive transport Diffusion: Facilitated diffusion ([Uniporter])
OsmosisActive transport -cytosis Exocytosis (Degranulation) - Endocytosis (Phagocytosis, transcytosis, fluid-phase pinocytosis, non-specific, adsorptive pinocytosis, Receptor-mediated endocytosis, Potocytosis, Efferocytosis)Categories:- Cellular processes
- Neurophysiology
- Greek loanwords
Wikimedia Foundation. 2010.

