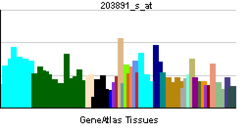
- DAPK3
-
Death-associated protein kinase 3 is an enzyme that in humans is encoded by the DAPK3 gene.[1][2]
Death-associated protein kinase 3 (DAPK3) induces morphological changes in apoptosis when overexpressed in mammalian cells. These results suggest that DAPK3 may play a role in the induction of apoptosis.[2]
Interactions
DAPK3 has been shown to interact with PAWR[3] and Death associated protein 6.[3]
References
- ^ Kawai T, Matsumoto M, Takeda K, Sanjo H, Akira S (Mar 1998). "ZIP kinase, a novel serine/threonine kinase which mediates apoptosis". Mol Cell Biol 18 (3): 1642–51. PMC 108879. PMID 9488481. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=108879.
- ^ a b "Entrez Gene: DAPK3 death-associated protein kinase 3". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1613.
- ^ a b Kawai, Taro; Akira Shizuo, Reed John C (Sep. 2003). "ZIP kinase triggers apoptosis from nuclear PML oncogenic domains". Mol. Cell. Biol. (United States) 23 (17): 6174–86. doi:10.1128/MCB.23.17.6174-6186.2003. ISSN 0270-7306. PMC 180930. PMID 12917339. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=180930.
Further reading
- Saito T, Seki N, Ohira M, et al. (1998). "Assignment of the ZIP kinase gene to human chromosome 19p13.3 by somatic hybrid analysis and fluorescence in-situ hybridization.". J. Hum. Genet. 43 (3): 209–11. doi:10.1007/s100380050073. PMID 9747039.
- Murata-Hori M, Suizu F, Iwasaki T, et al. (1999). "ZIP kinase identified as a novel myosin regulatory light chain kinase in HeLa cells.". FEBS Lett. 451 (1): 81–4. doi:10.1016/S0014-5793(99)00550-5. PMID 10356987.
- Page G, Lödige I, Kögel D, Scheidtmann KH (2000). "AATF, a novel transcription factor that interacts with Dlk/ZIP kinase and interferes with apoptosis.". FEBS Lett. 462 (1-2): 187–91. doi:10.1016/S0014-5793(99)01529-X. PMID 10580117.
- Page G, Kögel D, Rangnekar V, Scheidtmann KH (2000). "Interaction partners of Dlk/ZIP kinase: co-expression of Dlk/ZIP kinase and Par-4 results in cytoplasmic retention and apoptosis.". Oncogene 18 (51): 7265–73. doi:10.1038/sj.onc.1203170. PMID 10602480.
- Cariou B, Perdereau D, Cailliau K, et al. (2002). "The adapter protein ZIP binds Grb14 and regulates its inhibitory action on insulin signaling by recruiting protein kinase Czeta.". Mol. Cell. Biol. 22 (20): 6959–70. doi:10.1128/MCB.22.20.6959-6970.2002. PMC 139806. PMID 12242277. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139806.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences.". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139241.
- Preuss U, Landsberg G, Scheidtmann KH (2003). "Novel mitosis-specific phosphorylation of histone H3 at Thr11 mediated by Dlk/ZIP kinase.". Nucleic Acids Res. 31 (3): 878–85. doi:10.1093/nar/gkg176. PMC 149197. PMID 12560483. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=149197.
- Kawai T, Akira S, Reed JC (2003). "ZIP kinase triggers apoptosis from nuclear PML oncogenic domains.". Mol. Cell. Biol. 23 (17): 6174–86. doi:10.1128/MCB.23.17.6174-6186.2003. PMC 180930. PMID 12917339. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=180930.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs.". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Burch LR, Scott M, Pohler E, et al. (2004). "Phage-peptide display identifies the interferon-responsive, death-activated protein kinase family as a novel modifier of MDM2 and p21WAF1.". J. Mol. Biol. 337 (1): 115–28. doi:10.1016/j.jmb.2003.10.081. PMID 15001356.
- Endo A, Surks HK, Mochizuki S, et al. (2004). "Identification and characterization of zipper-interacting protein kinase as the unique vascular smooth muscle myosin phosphatase-associated kinase.". J. Biol. Chem. 279 (40): 42055–61. doi:10.1074/jbc.M403676200. PMID 15292222.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC).". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=528928.
- Schaaf CP, Benzing J, Schmitt T, et al. (2005). "Novel interaction partners of the TPR/MET tyrosine kinase.". FASEB J. 19 (2): 267–9. doi:10.1096/fj.04-1558fje. PMID 15546961.
- Yu H, Jiang D, Guo Z, et al. (2005). "TCP10L is expressed specifically in spermatogenic cells and binds to death associated protein kinase-3.". Int. J. Androl. 28 (3): 163–70. doi:10.1111/j.1365-2605.2005.00522.x. PMID 15910542.
- Shoval Y, Pietrokovski S, Kimchi A. (2007). "ZIPK: a unique case of murine-specific divergence of a conserved vertebrate gene.". PLoS Genet. 3 (10): 1884–93. doi:10.1371/journal.pgen.0030180. PMC 2041995. PMID 17953487. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2041995.
- Takamoto N, Komatsu S, Komaba S, et al. (2007). "Novel ZIP kinase isoform lacks leucine zipper.". Arch. Biochem. Biophys. 456 (2): 194–203. doi:10.1016/j.abb.2006.09.026. PMC 2758612. PMID 17126281. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2758612.
PDB gallery Kinases: Serine/threonine-specific protein kinases (EC 2.7.11-12) Serine/threonine-specific protein kinases (EC 2.7.11.1-EC 2.7.11.20) Non-specific serine/threonine protein kinases (EC 2.7.11.1)Pyruvate dehydrogenase kinase (EC 2.7.11.2)Dephospho-(reductase kinase) kinase (EC 2.7.11.3)(isocitrate dehydrogenase (NADP+)) kinase (EC 2.7.11.5)(tyrosine 3-monooxygenase) kinase (EC 2.7.11.6)Myosin-heavy-chain kinase (EC 2.7.11.7)Fas-activated serine/threonine kinase (EC 2.7.11.8)Goodpasture-antigen-binding protein kinase (EC 2.7.11.9)-IκB kinase (EC 2.7.11.10)cAMP-dependent protein kinase (EC 2.7.11.11)cGMP-dependent protein kinase (EC 2.7.11.12)Protein kinase C (EC 2.7.11.13)Rhodopsin kinase (EC 2.7.11.14)Beta adrenergic receptor kinase (EC 2.7.11.15)G-protein coupled receptor kinases (EC 2.7.11.16)Ca2+/calmodulin-dependent (EC 2.7.11.17)BRSK2, CAMK1, CAMK2A, CAMK2B, CAMK2D, CAMK2G, CAMK4, MLCK, CASK, CHEK1, CHEK2, DAPK1, DAPK2, DAPK3, STK11, MAPKAPK2, MAPKAPK3, MAPKAPK5, MARK1, MARK2, MARK3, MARK4, MELK, MKNK1, MKNK2, NUAK1, NUAK2, OBSCN, PASK, PHKG1, PHKG2, PIM1, PIM2, PKD1, PRKD2, PRKD3, PSKH1, SNF1LK2, KIAA0999, STK40, SNF1LK, SNRK, SPEG, TSSK2, Kalirin, TRIB1, TRIB2, TRIB3, TRIO, Titin, DCLK1Myosin light-chain kinase (EC 2.7.11.18)MYLK, MYLK2, MYLK3, MYLK4Phosphorylase kinase (EC 2.7.11.19)Elongation factor 2 kinase (EC 2.7.11.20)Serine/threonine-specific protein kinases (EC 2.7.11.21-EC 2.7.11.30) Polo kinase (EC 2.7.11.21)Cyclin-dependent kinase (EC 2.7.11.22)(RNA-polymerase)-subunit kinase (EC 2.7.11.23)Mitogen-activated protein kinase (EC 2.7.11.24)Extracellular signal-regulated (MAPK1, MAPK3, MAPK4, MAPK6, MAPK7, MAPK12, MAPK15), C-Jun N-terminal (MAPK8, MAPK9, MAPK10), P38 mitogen-activated protein (MAPK11, MAPK13, MAPK14)MAP3K (EC 2.7.11.25)Tau-protein kinase (EC 2.7.11.26)(acetyl-CoA carboxylase) kinase (EC 2.7.11.27)-Tropomyosin kinase (EC 2.7.11.28)-Low-density-lipoprotein receptor kinase (EC 2.7.11.29)-Receptor protein serine/threonine kinase (EC 2.7.11.30)Dual-specificity kinases (EC 2.7.12) Categories:- Human proteins
- Chromosome 19 gene stubs
- Cell signaling
- Signal transduction
Wikimedia Foundation. 2010.