- PDK2
-
Pyruvate dehydrogenase kinase isoform 2 (PDK2) also known as [pyruvate dehydrogenase [lipoamide]] kinase isozyme 2, mitochondrial is an enzyme that in humans is encoded by the PDK2 gene.[1][2] PDK2 is an isozyme of pyruvate dehydrogenase kinase.
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.[3]
Citric_acid_cycle edit
References
- ^ Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, Popov KM (Jan 1996). "Diversity of the pyruvate dehydrogenase kinase gene family in humans". J Biol Chem 270 (48): 28989–94. doi:10.1074/jbc.270.48.28989. PMID 7499431.
- ^ "Entrez Gene: PDK2 pyruvate dehydrogenase kinase, isozyme 2". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5164.
- ^ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78". http://www.wikipathways.org/index.php/Pathway:WP78.
Further reading
- Sugden MC, Holness MJ (2003). "Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs.". Am. J. Physiol. Endocrinol. Metab. 284 (5): E855–62. doi:10.1152/ajpendo.00526.2002. PMID 12676647.
- Kobayashi T, Cohen P (1999). "Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2.". Biochem. J. 339 ( Pt 2) (2): 319–28. doi:10.1042/0264-6021:3390319. PMC 1220160. PMID 10191262. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1220160.
- Gold MR, Scheid MP, Santos L, et al. (1999). "The B cell antigen receptor activates the Akt (protein kinase B)/glycogen synthase kinase-3 signaling pathway via phosphatidylinositol 3-kinase". J. Immunol. 163 (4): 1894–905. PMID 10438924.
- Baker JC, Yan X, Peng T, et al. (2000). "Marked differences between two isoforms of human pyruvate dehydrogenase kinase". J. Biol. Chem. 275 (21): 15773–81. doi:10.1074/jbc.M909488199. PMID 10748134.
- Steussy CN, Popov KM, Bowker-Kinley MM, et al. (2001). "Structure of pyruvate dehydrogenase kinase. Novel folding pattern for a serine protein kinase". J. Biol. Chem. 276 (40): 37443–50. doi:10.1074/jbc.M104285200. PMC 2147663. PMID 11483605. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2147663.
- Kolobova E, Tuganova A, Boulatnikov I, Popov KM (2001). "Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites". Biochem. J. 358 (Pt 1): 69–77. doi:10.1042/0264-6021:3580069. PMC 1222033. PMID 11485553. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1222033.
- Korotchkina LG, Patel MS (2001). "Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase". J. Biol. Chem. 276 (40): 37223–9. doi:10.1074/jbc.M103069200. PMID 11486000.
- Peters SJ, Harris RA, Wu P, et al. (2002). "Human skeletal muscle PDH kinase activity and isoform expression during a 3-day high-fat/low-carbohydrate diet". Am. J. Physiol. Endocrinol. Metab. 281 (6): E1151–8. PMID 11701428.
- Tuganova A, Boulatnikov I, Popov KM (2002). "Interaction between the individual isoenzymes of pyruvate dehydrogenase kinase and the inner lipoyl-bearing domain of transacetylase component of pyruvate dehydrogenase complex". Biochem. J. 366 (Pt 1): 129–36. doi:10.1042/BJ20020301. PMC 1222743. PMID 11978179. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1222743.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139241.
- Boulatnikov I, Popov KM (2003). "Formation of functional heterodimers by isozymes 1 and 2 of pyruvate dehydrogenase kinase". Biochim. Biophys. Acta 1645 (2): 183–92. doi:10.1016/S1570-9639(02)00542-3. PMID 12573248.
- Hiromasa Y, Roche TE (2003). "Facilitated interaction between the pyruvate dehydrogenase kinase isoform 2 and the dihydrolipoyl acetyltransferase". J. Biol. Chem. 278 (36): 33681–93. doi:10.1074/jbc.M212733200. PMID 12816949.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Watt MJ, Heigenhauser GJ, LeBlanc PJ, et al. (2005). "Rapid upregulation of pyruvate dehydrogenase kinase activity in human skeletal muscle during prolonged exercise". J. Appl. Physiol. 97 (4): 1261–7. doi:10.1152/japplphysiol.00132.2004. PMID 15169745.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=528928.
- Bao H, Kasten SA, Yan X, Roche TE (2004). "Pyruvate dehydrogenase kinase isoform 2 activity limited and further inhibited by slowing down the rate of dissociation of ADP". Biochemistry 43 (42): 13432–41. doi:10.1021/bi049488x. PMID 15491150.
- Bao H, Kasten SA, Yan X, et al. (2004). "Pyruvate dehydrogenase kinase isoform 2 activity stimulated by speeding up the rate of dissociation of ADP". Biochemistry 43 (42): 13442–51. doi:10.1021/bi0494875. PMID 15491151.
- Abbot EL, McCormack JG, Reynet C, et al. (2005). "Diverging regulation of pyruvate dehydrogenase kinase isoform gene expression in cultured human muscle cells". FEBS J. 272 (12): 3004–14. doi:10.1111/j.1742-4658.2005.04713.x. PMID 15955060.
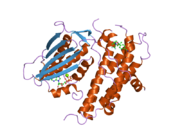
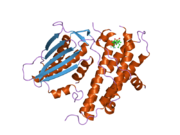
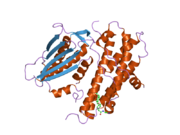
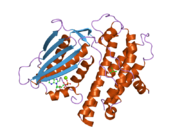
PDB gallery 1jm6: Pyruvate dehydrogenase kinase, isozyme 2, containing ADP2btz: CRYSTAL STRUCTURES OF HUMAN PYRUVATE DEHYDROGENASE KINASE 2 CONTAINING PHYSIOLOGICAL AND SYNTHETIC LIGANDS2bu2: CRYSTAL STRUCTURES OF HUMAN PYRUVATE DEHYDROGENASE KINASE 2 CONTAINING PHYSIOLOGICAL AND SYNTHETIC LIGANDS2bu5: CRYSTAL STRUCTURES OF HUMAN PYRUVATE DEHYDROGENASE KINASE 2 CONTAINING PHYSIOLOGICAL AND SYNTHETIC LIGANDS2bu6: CRYSTAL STRUCTURES OF HUMAN PYRUVATE DEHYDROGENASE KINASE 2 CONTAINING PHYSIOLOGICAL AND SYNTHETIC LIGANDS2bu7: CRYSTAL STRUCTURES OF HUMAN PYRUVATE DEHYDROGENASE KINASE 2 CONTAINING PHYSIOLOGICAL AND SYNTHETIC LIGANDS2bu8: CRYSTAL STRUCTURES OF HUMAN PYRUVATE DEHYDROGENASE KINASE 2 CONTAINING PHYSIOLOGICAL AND SYNTHETIC LIGANDSKinases: Serine/threonine-specific protein kinases (EC 2.7.11-12) Serine/threonine-specific protein kinases (EC 2.7.11.1-EC 2.7.11.20) Non-specific serine/threonine protein kinases (EC 2.7.11.1)Pyruvate dehydrogenase kinase (EC 2.7.11.2)Dephospho-(reductase kinase) kinase (EC 2.7.11.3)(isocitrate dehydrogenase (NADP+)) kinase (EC 2.7.11.5)(tyrosine 3-monooxygenase) kinase (EC 2.7.11.6)Myosin-heavy-chain kinase (EC 2.7.11.7)Fas-activated serine/threonine kinase (EC 2.7.11.8)Goodpasture-antigen-binding protein kinase (EC 2.7.11.9)-IκB kinase (EC 2.7.11.10)cAMP-dependent protein kinase (EC 2.7.11.11)cGMP-dependent protein kinase (EC 2.7.11.12)Protein kinase C (EC 2.7.11.13)Rhodopsin kinase (EC 2.7.11.14)Beta adrenergic receptor kinase (EC 2.7.11.15)G-protein coupled receptor kinases (EC 2.7.11.16)Ca2+/calmodulin-dependent (EC 2.7.11.17)BRSK2, CAMK1, CAMK2A, CAMK2B, CAMK2D, CAMK2G, CAMK4, MLCK, CASK, CHEK1, CHEK2, DAPK1, DAPK2, DAPK3, STK11, MAPKAPK2, MAPKAPK3, MAPKAPK5, MARK1, MARK2, MARK3, MARK4, MELK, MKNK1, MKNK2, NUAK1, NUAK2, OBSCN, PASK, PHKG1, PHKG2, PIM1, PIM2, PKD1, PRKD2, PRKD3, PSKH1, SNF1LK2, KIAA0999, STK40, SNF1LK, SNRK, SPEG, TSSK2, Kalirin, TRIB1, TRIB2, TRIB3, TRIO, Titin, DCLK1Myosin light-chain kinase (EC 2.7.11.18)MYLK, MYLK2, MYLK3, MYLK4Phosphorylase kinase (EC 2.7.11.19)Elongation factor 2 kinase (EC 2.7.11.20)Serine/threonine-specific protein kinases (EC 2.7.11.21-EC 2.7.11.30) Polo kinase (EC 2.7.11.21)Cyclin-dependent kinase (EC 2.7.11.22)(RNA-polymerase)-subunit kinase (EC 2.7.11.23)Mitogen-activated protein kinase (EC 2.7.11.24)Extracellular signal-regulated (MAPK1, MAPK3, MAPK4, MAPK6, MAPK7, MAPK12, MAPK15), C-Jun N-terminal (MAPK8, MAPK9, MAPK10), P38 mitogen-activated protein (MAPK11, MAPK13, MAPK14)MAP3K (EC 2.7.11.25)Tau-protein kinase (EC 2.7.11.26)(acetyl-CoA carboxylase) kinase (EC 2.7.11.27)-Tropomyosin kinase (EC 2.7.11.28)-Low-density-lipoprotein receptor kinase (EC 2.7.11.29)-Receptor protein serine/threonine kinase (EC 2.7.11.30)Dual-specificity kinases (EC 2.7.12) Categories:- Human proteins
- Cell signaling
- Signal transduction
- Chromosome 17 gene stubs
- EC 2.7.11
Wikimedia Foundation. 2010.