- PAK3
-
P21 protein (Cdc42/Rac)-activated kinase 3 
PDB rendering based on 1e0a.Available structures PDB 1e0a, 1ees, 1f3m, 1yhv, 1yhw, 2hy8 Identifiers Symbols PAK3; CDKN1A; MRX30; MRX47; OPHN3; PAK3beta; bPAK; hPAK3 External IDs OMIM: 300142 MGI: 1339656 HomoloGene: 55664 GeneCards: PAK3 Gene EC number 2.7.11.1 Gene Ontology Molecular function • nucleotide binding
• protein serine/threonine kinase activity
• ATP binding
• SH3 domain binding
• metal ion bindingBiological process • multicellular organismal development Sources: Amigo / QuickGO RNA expression pattern 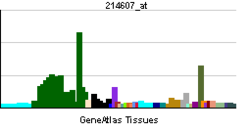
More reference expression data Orthologs Species Human Mouse Entrez 5063 18481 Ensembl ENSG00000077264 ENSMUSG00000031284 UniProt O75914 Q61036 RefSeq (mRNA) NM_001128166.1 NM_008778 RefSeq (protein) NP_001121638.1 NP_032804 Location (UCSC) Chr X:
110.19 – 110.46 MbChr X:
139.95 – 140.23 MbPubMed search [1] [2] Serine/threonine-protein kinase PAK 3 is an enzyme that in humans is encoded by the PAK3 gene.[1][2][3]
PAK proteins are critical effectors that link Rho GTPases to cytoskeleton reorganization and nuclear signaling. PAK proteins, a family of serine/threonine p21-activating kinases, serve as targets for the small GTP binding proteins Cdc42 and RAC and have been implicated in a wide range of biological activities. The protein encoded by this gene forms an activated complex with GTP-bound RAS-like (P21), CDC2 and RAC1 proteins which then catalyzes a variety of targets. This protein may be necessary for dendritic development and for the rapid cytoskeletal reorganization in dendritic spines associated with synaptic plasticity. A point mutation in this gene has been linked to nonsyndromic X-linked mental retardation.[3]
Contents
Interactions
PAK3 has been shown to interact with RAC1.[4]
References
- ^ Donnelly AJ, Partington MW, Ryan AK, Mulley JC (Nov 1996). "Regional localisation of two non-specific X-linked mental retardation genes (MRX30 and MRX31)". Am J Med Genet 64 (1): 113–20. doi:10.1002/(SICI)1096-8628(19960712)64:1<113::AID-AJMG19>3.0.CO;2-Q. PMID 8826460.
- ^ Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, Cerione RA, Mulley JC, Walsh CA (Oct 1998). "PAK3 mutation in nonsyndromic X-linked mental retardation". Nat Genet 20 (1): 25–30. doi:10.1038/1675. PMID 9731525.
- ^ a b "Entrez Gene: PAK3 p21 (CDKN1A)-activated kinase 3". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5063.
- ^ Shin, O H; Exton J H (Aug. 2001). "Differential binding of arfaptin 2/POR1 to ADP-ribosylation factors and Rac1". Biochem. Biophys. Res. Commun. (United States) 285 (5): 1267–73. doi:10.1006/bbrc.2001.5330. ISSN 0006-291X. PMID 11478794.
Further reading
- Bagrodia S, Cerione RA (1999). "Pak to the future". Trends Cell Biol. 9 (9): 350–5. doi:10.1016/S0962-8924(99)01618-9. PMID 10461188.
- Illarioshkin SN, Tanaka H, Markova ED, et al. (1996). "X-linked nonprogressive congenital cerebellar hypoplasia: clinical description and mapping to chromosome Xq". Ann. Neurol. 40 (1): 75–83. doi:10.1002/ana.410400113. PMID 8687195.
- Siow YL, Kalmar GB, Sanghera JS, et al. (1997). "Identification of two essential phosphorylated threonine residues in the catalytic domain of Mekk1. Indirect activation by Pak3 and protein kinase C". J. Biol. Chem. 272 (12): 7586–94. doi:10.1074/jbc.272.12.7586. PMID 9065412.
- Diaz B, Barnard D, Filson A, et al. (1997). "Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling". Mol. Cell. Biol. 17 (8): 4509–16. PMC 232304. PMID 9234708. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=232304.
- des Portes V, Soufir N, Carrié A, et al. (1997). "Gene for nonspecific X-linked mental retardation (MRX 47) is located in Xq22.3-q24". Am. J. Med. Genet. 72 (3): 324–8. doi:10.1002/(SICI)1096-8628(19971031)72:3<324::AID-AJMG14>3.0.CO;2-V. PMID 9332663.
- Manser E, Loo TH, Koh CG, et al. (1998). "PAK kinases are directly coupled to the PIX family of nucleotide exchange factors". Mol. Cell 1 (2): 183–92. doi:10.1016/S1097-2765(00)80019-2. PMID 9659915.
- Van Eyk JE, Arrell DK, Foster DB, et al. (1998). "Different molecular mechanisms for Rho family GTPase-dependent, Ca2+-independent contraction of smooth muscle". J. Biol. Chem. 273 (36): 23433–9. doi:10.1074/jbc.273.36.23433. PMID 9722579.
- Bagrodia S, Taylor SJ, Jordon KA, et al. (1998). "A novel regulator of p21-activated kinases". J. Biol. Chem. 273 (37): 23633–6. doi:10.1074/jbc.273.37.23633. PMID 9726964.
- King AJ, Sun H, Diaz B, et al. (1998). "The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338". Nature 396 (6707): 180–3. doi:10.1038/24184. PMID 9823899.
- Mason CS, Springer CJ, Cooper RG, et al. (1999). "Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation". EMBO J. 18 (8): 2137–48. doi:10.1093/emboj/18.8.2137. PMC 1171298. PMID 10205168. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1171298.
- Premont RT, Claing A, Vitale N, et al. (2000). "The GIT family of ADP-ribosylation factor GTPase-activating proteins. Functional diversity of GIT2 through alternative splicing". J. Biol. Chem. 275 (29): 22373–80. doi:10.1074/jbc.275.29.22373. PMID 10896954.
- Bienvenu T, des Portes V, McDonell N, et al. (2001). "Missense mutation in PAK3, R67C, causes X-linked nonspecific mental retardation". Am. J. Med. Genet. 93 (4): 294–8. doi:10.1002/1096-8628(20000814)93:4<294::AID-AJMG8>3.0.CO;2-F. PMID 10946356.
- Hashimoto S, Tsubouchi A, Mazaki Y, Sabe H (2001). "Interaction of paxillin with p21-activated Kinase (PAK). Association of paxillin alpha with the kinase-inactive and the Cdc42-activated forms of PAK3". J. Biol. Chem. 276 (8): 6037–45. doi:10.1074/jbc.M005854200. PMID 11096073.
- Chiloeches A, Mason CS, Marais R (2001). "S338 phosphorylation of Raf-1 is independent of phosphatidylinositol 3-kinase and Pak3". Mol. Cell. Biol. 21 (7): 2423–34. doi:10.1128/MCB.21.7.2423-2434.2001. PMC 86875. PMID 11259591. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=86875.
- Chong C, Tan L, Lim L, Manser E (2001). "The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity". J. Biol. Chem. 276 (20): 17347–53. doi:10.1074/jbc.M009316200. PMID 11278486.
- Stoletov KV, Ratcliffe KE, Spring SC, Terman BI (2001). "NCK and PAK participate in the signaling pathway by which vascular endothelial growth factor stimulates the assembly of focal adhesions". J. Biol. Chem. 276 (25): 22748–55. doi:10.1074/jbc.M009720200. PMID 11278553.
- Yoshimura M, Homma K, Saito J, et al. (2001). "Dual regulation of mammalian myosin VI motor function". J. Biol. Chem. 276 (43): 39600–7. doi:10.1074/jbc.M105080200. PMID 11517222.
External links
- PAK3 Info with links in the Cell Migration Gateway
PDB gallery 1e0a: CDC42 COMPLEXED WITH THE GTPASE BINDING DOMAIN OF P21 ACTIVATED KINASE1ees: SOLUTION STRUCTURE OF CDC42HS COMPLEXED WITH A PEPTIDE DERIVED FROM P-21 ACTIVATED KINASE, NMR, 20 STRUCTURES1f3m: CRYSTAL STRUCTURE OF HUMAN SERINE/THREONINE KINASE PAK11yhv: Crystal Structure of PAK1 kinase domain with two point mutations (K299R, T423E)1yhw: Crystal Structure of PAK1 kinase domain with one point mutations (K299R)2hy8: PAK1 complex with ST2001Categories:- Human proteins
- Chromosome X gene stubs
Wikimedia Foundation. 2010.