- Esomeprazole
-
Esomeprazole 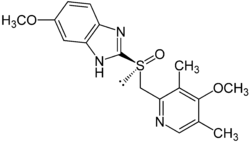
Systematic (IUPAC) name (S)-5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)
methylsulfinyl]-3H-benzoimidazoleClinical data Trade names Nexium AHFS/Drugs.com monograph MedlinePlus a699054 Pregnancy cat. B3(AU) B(US) Legal status Prescription Only (S4) (AU) POM (UK) ℞-only (US) Routes Oral, IV Pharmacokinetic data Bioavailability 50 to 90% Metabolism Hepatic (CYP2C19, CYP3A4) Half-life 1–1.5 hours Excretion 80% Renal
20% FaecalIdentifiers CAS number 119141-88-7 
ATC code A02BC05 PubChem CID 9579578 DrugBank APRD00363 ChemSpider 7853936 
UNII N3PA6559FT 
KEGG D07917 
ChEBI CHEBI:50275 
ChEMBL CHEMBL1201320 
Chemical data Formula C17H19N3O3S Mol. mass 345.417 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Esomeprazole (
 /iːsɵˈmɛprəzoʊl/) is a proton pump inhibitor (brand name Nexium) developed and marketed by AstraZeneca which is used in the treatment of dyspepsia, peptic ulcer disease (PUD), gastroesophageal reflux disease (GORD/GERD) and Zollinger-Ellison syndrome. Esomeprazole is the S-enantiomer of omeprazole (marketed as Losec/Prilosec), and AstraZeneca claims improved efficacy of this single enantiomer product over the racemic mixture of omeprazole. However, this greater efficacy has been disputed, with some claiming it offers no benefit from its older form.
/iːsɵˈmɛprəzoʊl/) is a proton pump inhibitor (brand name Nexium) developed and marketed by AstraZeneca which is used in the treatment of dyspepsia, peptic ulcer disease (PUD), gastroesophageal reflux disease (GORD/GERD) and Zollinger-Ellison syndrome. Esomeprazole is the S-enantiomer of omeprazole (marketed as Losec/Prilosec), and AstraZeneca claims improved efficacy of this single enantiomer product over the racemic mixture of omeprazole. However, this greater efficacy has been disputed, with some claiming it offers no benefit from its older form.Esomeprazole is a proton pump inhibitor which reduces acid secretion through inhibition of ATPase in gastric parietal cells. By inhibiting the functioning of this enzyme, the drug prevents formation of gastric acid.
Contents
Medical use
The primary uses of esomeprazole are gastroesophageal reflux disease, treatment of duodenal ulcers caused by H. pylori, preventing of gastric ulcers in those on chronic NSAID therapy, and treatment of gastrointestinal ulcers associated with Crohn's disease.[1]
Gastroesophageal reflux disease
Gastroesophageal Reflux Disease (GERD) is a condition in which the digestive acid in the stomach comes in contact with the oesophagus (food pipe). The irritation caused by this disorder is known as heartburn. Long term contact between the acid and esophagus can cause permanent damage to the esophagus. Esomeprazole (Nexium) reduces the production of digestives acids, thus minimizing their effect on the esophagus.
Duodenal ulcers
Esomeprazole is combined with the antibiotics clarithromycin and amoxicillin (or metronidazole in penicillin-hypersensitive patients) in the 7-14 day eradication triple therapy for Helicobacter pylori. Infection by H. pylori is the causative factor in the majority of peptic and duodenal ulcers.
Evidence of efficacy
AstraZeneca claims that esomeprazole provides improved efficacy, in terms of stomach acid control, over the R enantiomer of omeprazole. Many health professionals have expressed the view that this improvement in efficacy is due to the dose of esomeprazole recommended for therapy rather than any inherent superiority of esomeprazole.
An alternative rationale suggested for the use of esomeprazole was the reduction in interindividual variability in efficacy. However the clinical advantage of this hypothesis has not thoroughly been tested in large-scale trials.[2]
Given the large difference in cost between all other proton pump inhibitors and that of omeprazole, many physicians recommend a trial of over-the-counter products before beginning more extensive therapies and testing.
Although the (S)-isomer is more potent in humans, the (R)-isomer is more potent in the testing of rats, while the enantiomers are equipotent in dogs.[3]
Adverse effects
Common side effects include headache, diarrhea, nausea, gas, decreased appetite, constipation, dry mouth, and abdominal pain. More severe side effects are severe allergic reactions, chest pain, dark urine, fast heartbeat, fever, paresthesia, persistent sore throat, severe stomach pain, unusual bruising or bleeding, unusual tiredness, and yellowing of the eyes or skin.[4]
Proton pump inhibitors may be associated with a greater risk of hip fractures[5] and clostridium difficile-associated diarrhea.[6] Patients are frequently administered the drugs in intensive care as a protective measure against ulcers, but this use is also associated with a 30% increase in occurrence of pneumonia.[7]
Interactions
Esomeprazole is a competitive inhibitor of the enzymes CYP2C19 and CYP2C9, and may therefore interact with drugs that depend on them for metabolism, such as diazepam and warfarin; the concentrations of these drugs may increase if they are used concomitantly with esomeprazole.[8] Conversely, Clopidogrel (Plavix) is an inactive prodrug that partially depends on CYP2C19 for conversion to its active form; inhibition of CYP2C19 blocks the activation of clopidogrel, thus reducing its effects.[9][10]
Drugs that depend on stomach pH for absorption may interact with omeprazole; drugs that depend on an acidic environment (such as ketoconazole or atazanavir) will be poorly absorbed, whereas drugs that are broken down in acidic environments (such as erythromycin) will be absorbed to a greater extent than normal.[8]
Pharmacokinetics
Single 20-40 mg oral doses generally give rise to peak plasma esomeprazole concentrations of 0.5-1.0 mg/L within 1-4 hours, but after several days of once-daily administration these levels may increase by about 50%. A 30 minute intravenous infusion of a similar dose usually produces peak plasma levels on the order of 1-3 mg/L. The drug is rapidly cleared from the body, largely by urinary excretion of pharmacologically-inactive metabolites such as 5-hydroxymethylesomeprazole and 5-carboxyesomeprazole. Esomeprazole and its metabolites are analytically indistinguishable from omeprazole and the corresponding omeprazole metabolites unless chiral techniques are employed.[11]
Dosage forms
Esomeprazole is available as delayed-release capsules in the United States or as delayed release tablets in Australia and Canada (containing esomeprazole magnesium) in strengths of 20 mg and 40 mg; and as esomeprazole sodium for intravenous injection/infusion. Oral esomeprazole preparations are enteric-coated, due to the rapid degradation of the drug in the acidic conditions of the stomach. This is achieved by formulating capsules using the multiple-unit pellet system.
Multiple unit pellet system
Esomeprazole capsules are formulated as a "multiple unit pellet system" (MUPS). Essentially, the capsule consists of extremely small enteric-coated granules (pellets) of the esomeprazole formulation inside an outer shell. When the capsule is immersed in an aqueous solution, as happens when the capsule reaches the stomach, water enters the capsule by osmosis. The contents swell from water absorption causing the shell to burst, releasing the enteric-coated granules. For most patients, the multiple-unit pellet system is of no advantage over conventional enteric-coated preparations. Patients for which the formulation is of benefit include those requiring nasogastric tube feeding and those with difficulty swallowing (dysphagia).
Society and culture
The granules are manufactured in a fluid bed system with small sugar spheres as the starting material. The sugar spheres are sequentially spray-coated with a suspension containing esomeprazole, a protective layer to prevent degradation of the drug in manufacturing, an enteric coating and an outer layer to reduce granule aggregation. The granules are mixed with other inactive excipients and compressed into tablets. Finally, the tablets are film-coated to improve the stability and appearance of the preparation.
Economics
Between the launch of esomeprazole in 2001 and 2005, the drug has netted AstraZeneca about $14.4 billion.[12]
Controversy
There has been some controversy about AstraZeneca's behaviour in creating, patenting and marketing of the drug. Esomeprazole's successful predecessor omeprazole is a mixture of two mirror-imaged molecules (esomeprazole which is the S-enantiomer, and R-omeprazole), and that the company was trying to "evergreen" its patent by patenting the pure esomeprazole and aggressively marketing to doctors that it is more effective than the mixture,[13] claiming that omeprazole has no beneficial effects on the patient. However, in the acidic environment of the parietal cells both esomeprazole and omeprazole are converted to the same active drug which stops the gastric acid production.
Dr. Marcia Angell, former Editor-in-Chief of the New England Journal of Medicine, spoke at Harvard Medical School to a German magazine on August 16, 2007 and accused AstraZeneca's scientists of deceptively doctoring their comparative studies such that the difference to Omeprazole would look larger, providing a marketing advantage.[14] For more information, see AstraZeneca's article.
Thomas A. Scully, head of the Federal Centers for Medicare and Medicaid services also criticized AstraZeneca for their aggressive marketing of Nexium. At a conference of the American Medical Association he went so far as to suggest that Astra was using the new drug to overcharge consumers and insurance companies. "You should be embarrassed if you prescribe Nexium," he claimed, "because you're screwing your patients and you're screwing the taxpayers."[citation needed] An AstraZeneca sponsored study showed that 40 mg of esomeprazole provided more effective acid control than 40 mg of omeprazole,[15] however this has not been borne out in large scale trials in humans and may be a result of the dosage formulation of Nexium.[2]
Brand names
Brand names include Zoleri, Nexium,Lucen, Esopral; Axagon in Italy, Nexiam in Belgium and South Africa; Sompraz and Esomac in India
References
- ^ "Esomeprazole Magnesium". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/esomeprazole-magnesium.html. Retrieved 3 April 2011.
- ^ a b Somogyi, A; Bochner, F; Foster, D (2004). "Inside the isomers: the tale of chiral switches". Australian Prescriber (National Prescribing Service) 27 (2): 47–9. http://www.australianprescriber.com/magazine/27/2/47/9/. Retrieved 2009-06-23.
- ^ Silverman, Richard B. (2004). "3: Receptors". The organic chemistry of drug design and drug action (2nd ed.). Academic Press. p. 148. ISBN 978-0-12-643732-4. http://books.google.co.uk/books?id=uc0e-Nbkh4oC&dq=The+Organic+Chemistry+of+Drug+Design+and+Drug+Action&printsec=frontcover&source=bn&hl=en&ei=D8dASrrQLKCUjAfd8IGSCQ&sa=X&oi=book_result&ct=result&resnum=4. Retrieved 2009-06-23.
- ^ "Nexium side effects". Drug information online. Drugs.com. http://www.drugs.com/sfx/nexium-side-effects.html. Retrieved 2009-06-23.
- ^ Yang YX, Lewis JD, Epstein S, Metz DC (2006). "Long-term Proton Pump Inhibitor Therapy and Risk of Hip Fracture". JAMA 296 (24): 2947–53. doi:10.1001/jama.296.24.2947. PMID 17190895.
- ^ "Proton pump inhibitors and Clostridium difficile". Bandolier. 2003. http://www.medicine.ox.ac.uk/bandolier/booth/Pharmacy/PPIcdiff.html. Retrieved 2007-07-13.
- ^ Shoshana J. Herzig, MD; Michael D. Howell, MD, MPH; Long H. Ngo, PhD; Edward R. Marcantonio, MD, SM (2009). "Acid-Suppressive Medication Use and the Risk for Hospital-Acquired Pneumonia=JAMA". JAMA the Journal of the American Medical Association 301 (20): 2120–2128. doi:10.1001/jama.2009.722. PMID 19470989. http://jama.ama-assn.org/cgi/content/abstract/301/20/2120?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&fulltext=shoshana+herzig&searchid=1&FIRSTINDEX=0&resourcetype=HWCIT.
- ^ a b Stedman CA, Barclay ML (August 2000). "Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors". Aliment Pharmacol Ther 14 (8): 963–78. doi:10.1046/j.1365-2036.2000.00788.x. PMID 10930890.
- ^ Lau WC, Gurbel PA (March 2009). "The drug–drug interaction between proton pump inhibitors and clopidogrel". CMAJ 180 (7): 699–700. doi:10.1503/cmaj.090251. PMC 2659824. PMID 19332744. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2659824.
- ^ Norgard NB, Mathews KD, Wall GC (July 2009). "Drug-drug interaction between clopidogrel and the proton pump inhibitors". Ann Pharmacother 43 (7): 1266–74. doi:10.1345/aph.1M051. PMID 19470853.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 388-389.
- ^ Financial impact information: 2005, $4.6 billion; 2004, $3.9 billion; 2003, $3.3 billion; 2002, $2 billion; 2001, launch and $580 million.
- ^ Gladwell, Malcolm (2004-10-25). "High Prices: How to think about prescription drugs". The New Yorker. http://www.newyorker.com/archive/2004/10/25/041025crat_atlarge. Retrieved 2006-06-23.
- ^ von Markus, Grill (2007-08-14). "Vorsicht, Pharma - Wie die Industrie Ärzte manipuliert und Patienten täuscht" (in German). Der Stern. http://www.stern.de/wirtschaft/unternehmen/unternehmen/:Pharmaindustrie-Vorsicht,-Pharma-Wie-Industrie-%C4rzte-Patienten/595277.html. Retrieved 2009-06-23.
- ^ Röhss K, Hasselgren G, Hedenström H (May 2002). "Effect of esomeprazole 40 mg vs omeprazole 40 mg on 24-hour intragastric pH in patients with symptoms of gastroesophageal reflux disease". Digestive Diseases and Sciences 47 (5): 954–8. doi:10.1023/A:1015009300955. PMID 12018920.
External links
- Nexium official site
- Details for Nexium
- U.S. National Library of Medicine: Drug Information Portal - Esomeprazole
AstraZeneca Products Anastrozole · Atenolol · Brompheniramine · Budesonide · Disufenton sodium · Esomeprazole · FluMist · Gefitinib · Goserelin · Isosorbide mononitrate · Motavizumab · Omeprazole · Palivizumab · Propofol · Rosuvastatin · Tamoxifen · Ticagrelor · Vandetanib · Ximelagatran · ZolmitriptanPredecessors and
acquired companiesPeople  CategoryCategories:
CategoryCategories:- Proton pump inhibitors
- AstraZeneca
- Benzimidazoles
- Sulfoxides
- Pyridines
- Enantiopure drugs
- Phenol ethers
- 2001 introductions
Wikimedia Foundation. 2010.

