- Diazepam
-
Diazepam 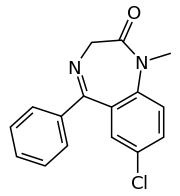
Systematic (IUPAC) name 7-chloro-1,3-dihydro-
1-methyl-5-phenyl-
1,4-benzodiazepin-2(3H)-oneClinical data Trade names Diastat, Valium AHFS/Drugs.com monograph MedlinePlus a682047 Pregnancy cat. C(AU) D(US) Legal status Prescription Only (S4) (AU) Schedule IV (CA) CD (UK) Schedule IV (US) Schedule IV (International) Routes Oral, IM, IV, suppository Pharmacokinetic data Bioavailability 93% Metabolism Hepatic - CYP2C19 Half-life 20–100 hours (36-200 hours for main active metabolite desmethyldiazepam) Excretion Renal Identifiers CAS number 439-14-5 
ATC code N05BA01 N05BA17 PubChem CID 3016 DrugBank DB00829 ChemSpider 2908 
UNII Q3JTX2Q7TU 
KEGG D00293 
ChEBI CHEBI:49575 
ChEMBL CHEMBL12 
Chemical data Formula C16H13ClN2O Mol. mass 284.7 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Diazepam (
 /daɪˈæzɨpæm/), first marketed as Valium (
/daɪˈæzɨpæm/), first marketed as Valium ( /ˈvæliəm/) by Hoffmann-La Roche is a benzodiazepine drug. Diazepam is also marketed in Australia as Antenex. It is commonly used for treating anxiety, insomnia, seizures including status epilepticus, muscle spasms (such as in cases of tetanus), restless legs syndrome, alcohol withdrawal, benzodiazepine withdrawal and Ménière's disease. It may also be used before certain medical procedures (such as endoscopies) to reduce tension and anxiety, and in some surgical procedures to induce amnesia.[1][2] It possesses anxiolytic, anticonvulsant, hypnotic, sedative, skeletal muscle relaxant, and amnestic properties.[3] The pharmacological action of diazepam enhances the effect of the neurotransmitter GABA by binding to the benzodiazepine site on the GABAA receptor leading to central nervous system depression.[4]
/ˈvæliəm/) by Hoffmann-La Roche is a benzodiazepine drug. Diazepam is also marketed in Australia as Antenex. It is commonly used for treating anxiety, insomnia, seizures including status epilepticus, muscle spasms (such as in cases of tetanus), restless legs syndrome, alcohol withdrawal, benzodiazepine withdrawal and Ménière's disease. It may also be used before certain medical procedures (such as endoscopies) to reduce tension and anxiety, and in some surgical procedures to induce amnesia.[1][2] It possesses anxiolytic, anticonvulsant, hypnotic, sedative, skeletal muscle relaxant, and amnestic properties.[3] The pharmacological action of diazepam enhances the effect of the neurotransmitter GABA by binding to the benzodiazepine site on the GABAA receptor leading to central nervous system depression.[4]Adverse effects of diazepam include anterograde amnesia (especially at higher doses) and sedation as well as paradoxical effects such as excitement, rage or worsening of seizures in epileptics. Benzodiazepines also can cause or worsen depression. Long-term effects of benzodiazepines such as diazepam include tolerance, benzodiazepine dependence as well as a benzodiazepine withdrawal syndrome upon dose reduction; additionally after cessation of benzodiazepines cognitive deficits may persist for at least 6 months and may not fully return to normal, however it was suggested that longer than 6 months may be needed for recovery from some deficits.[4] Diazepam also has physical dependence potential and can cause serious problems of physical dependence with long term use. However, compared to other benzodiazepines, physical withdrawal from diazepam following long term use is usually far more mild due to its long elimination half life. Nevertheless, urgent action by National Governments to improve prescribing practices has been recommended.[5]
Advantages of diazepam are a rapid onset of action and high efficacy rates which is important for managing acute seizures; benzodiazepines also have a relatively low toxicity in overdose.[4] Diazepam is a core medicine in the World Health Organization's "Essential Drugs List", which is a list of minimum medical needs for a basic health care system.[6] Diazepam is used to treat a wide range of conditions and has been one of the most frequently prescribed medications in the world for the past forty years. It was first synthesized by Leo Sternbach.[7]
Contents
Medical uses
 Diazepam pills, (2mg, 5mg, and 10mg)
Diazepam pills, (2mg, 5mg, and 10mg)
Diazepam is mainly used to treat anxiety, insomnia, and symptoms of acute alcohol withdrawal. It is also used as a premedication for inducing sedation, anxiolysis or amnesia before certain medical procedures (e.g., endoscopy).[8][9]
Intravenous diazepam or lorazepam are first line treatments for status epilepticus;[4][10] However, lorazepam has advantages over diazepam including a higher rate of terminating seizures and a more prolonged anticonvulsant effect.[11] Diazepam is rarely used for the long-term treatment of epilepsy because tolerance to the anticonvulsant effects of diazepam usually develops within 6 to 12 months of treatment, effectively rendering it useless for that purpose.[12][13] Diazepam is used for the emergency treatment of eclampsia, when IV magnesium sulfate and blood pressure control measures have failed.[14][15] Benzodiazepines do not have any pain relieving properties of themselves and are generally recommended to be avoided in individuals with pain.[16] However, benzodiazepines such as diazepam can be used for their muscle relaxant properties to alleviate pain which is caused by muscle spasms, caused by various dystonias, including blepharospasm[17][18] Tolerance often develops to the muscle relaxant effects of benzodiazepines such as diazepam.[19] Baclofen[20] or tizanidine is sometimes used as an alternative to diazepam. Tizanidine has been found to be equally effective as other antispasmodic drugs and have superior tolerability than baclofen and diazepam.[21]
The anticonvulsant effects of diazepam, can help in the treatment of seizures, due to a drug overdose or chemical toxicity as a result of exposure to sarin, VX, soman (or other organophosphate poisons; See #CANA), lindane, chloroquine, physostigmine, or pyrethroids[12][22] Diazepam is sometimes used intermittently for the prophylaxis of febrile seizures which occur as a result of a high fever in children and neonates under 5 years of age.[4][23] Long-term use of diazepam for the management of epilepsy is not recommended; however, a subgroup individuals with treatment resistant epilepsy benefit from long-term benzodiazepines and for such individuals clorazepate has been recommended due to its slower onset of tolerance to the anticonvulsant effects.[4]
Diazepam has a broad spectrum of indications (most of which are off-label), including:
- Treatment of anxiety, panic attacks, and states of agitation[8]
- Treatment of neurovegetative symptoms associated with vertigo[24]
- Treatment of the symptoms of alcohol, opiate and benzodiazepine withdrawal[8][25]
- Short-term treatment of insomnia[8]
- Treatment of tetanus, together with other measures of intensive-treatment[26]
- Adjunctive treatment of spastic muscular paresis (para-/tetraplegia) caused by cerebral or spinal cord conditions such as stroke, multiple sclerosis, spinal cord injury (long-term treatment is coupled with other rehabilitative measures)[27]
- Palliative treatment of stiff person syndrome[28]
- Pre-/postoperative sedation, anxiolysis and/or amnesia (e.g., before endoscopic or surgical procedures)[27]
- Treatment of complications with a hallucinogen crisis and stimulant overdoses and psychosis, such as LSD, cocaine, or methamphetamine.[12]
- Prophylactic treatment of oxygen toxicity during hyperbaric oxygen therapy[29]
Dosages should be determined on an individual basis, depending upon the condition to be treated, the severity of symptoms, the body weight of the patient, and any comorbid conditions the patient may have.[12]
Availability
Diazepam is marketed in over 500 brands throughout the world.[30] It is supplied in oral, injectable, inhalation and rectal forms.[12][31][32]
The United States military employs a specialized diazepam preparation known as CANA (Convulsive Antidote, Nerve Agent), which contains a mixture of diazepam, atropine and pralidoxime (2-PAM). One CANA kit is typically issued to service members, along with three Mark I NAAK kits, when operating in circumstances where chemical weapons in the form of nerve agents are considered a potential hazard. Both of these kits deliver drugs using auto-injectors. They are intended for use in "buddy aid" or "self aid" administration of the drugs in the field prior to decontamination and delivery of the patient to definitive medical care.[33]
Contraindications
Use of diazepam should be avoided, when possible, in individuals with the following conditions:[34]
- Ataxia.
- Severe hypoventilation.
- Acute narrow-angle glaucoma.
- Severe hepatic deficiencies (hepatitis and liver cirrhosis decrease elimination by a factor of 2).
- Severe renal deficiencies (for example, patients on dialysis).
- Liver disorders.
- Severe respiratory disorders.
- Severe sleep apnea.
- Severe depression, particularly when accompanied by suicidal tendencies.
- Psychosis.
- Pregnancy or breast feeding.
- Caution required in elderly or debilitated patients.
- Coma or shock.
- Abrupt discontinuation of therapy.
- Acute intoxication with alcohol, narcotics, or other psychoactive substances (with the exception of some hallucinogens, where it is occasionally used as a treatment for overdose).
- History of alcohol or drug dependence.
- Myasthenia gravis, or MG, an autoimmune disorder causing marked fatiguability.
- Hypersensitivity or allergy to any drug in the benzodiazepine class.
Special caution needed
- Benzodiazepines require special precaution if used in the alcohol- or drug-dependent individuals and individuals with comorbid psychiatric disorders.[35]
- Pediatric patients
- Less than 18 years of age – Treatment usually not indicated, except treatment of epilepsy, and pre-/postoperative treatment. The smallest possible effective dose should be used for this group of patients.[36]
- Under 6 months of age – Safety and effectiveness have not been established; diazepam should not be given to individuals in this age group.[28][36]
- Elderly and very ill patients – Possibility that apnea and/or cardiac arrest may occur. Concomitant use of other central nervous system depressants increases this risk. The smallest possible effective dose should be used for this group of patients.[28][36][37] The elderly metabolise benzodiazepines much more slowly than younger adults and are also more sensitive to the effects of benzodiazepines even at similar blood plasma levels. Doses of diazepam are recommended to be about half of those given to younger individuals and treatment limited to a maximum of 2 weeks. Long-acting benzodiazepines such as diazepam are not recommended for the elderly.[4] Diazepam may also be dangerous in geriatric patients owing to a significant increased risk of falls.[38]
- I.V. or I.M. injections in hypotensive individuals or those in shock should be administered carefully and vital signs should be monitored.[37]
- Benzodiazepines such as diazepam are lipophilic and rapidly penetrate membranes, and, therefore, rapidly cross over into the placenta with significant uptake of the drug. Use of benzodiazepines including diazepam in late pregnancy, especially high doses, may result in floppy infant syndrome.[39]
Pregnancy
Diazepam when taken late in pregnancy, during the third trimester, causes a definite risk of a severe benzodiazepine withdrawal syndrome in the neonate with symptoms including hypotonia, and reluctance to suck, to apnoeic spells, cyanosis, and impaired metabolic responses to cold stress. Floppy infant syndrome and sedation in the newborn may also occur. Symptoms of floppy infant syndrome and the neonatal benzodiazepine withdrawal syndrome have been reported to persist from hours to months after birth.[40]
Adverse effects
Adverse effects of benzodiazepines such as diazepam include anterograde amnesia and confusion (especially pronounced in higher doses) and sedation. The elderly are more prone to adverse effects of diazepam such as confusion, amnesia, ataxia and hangover effects as well as falls. Long-term use of benzodiazepines such as diazepam is associated with tolerance, benzodiazepine dependence as well as a benzodiazepine withdrawal syndrome.[4] Like other benzodiazepines, diazepam can impair short-term memory and learning of new information. While benzodiazepine drugs such as diazepam can cause anterograde amnesia, they do not cause retrograde amnesia; information learned before benzodiazepines is not impaired. Tolerance to the cognitive impairing effects of benzodiazepines does not tend to develop with long-term use. The elderly are more sensitive to the cognitive impairing effects of benzodiazepines.[41] Additionally after cessation of benzodiazepines cognitive deficits may persist for at least six months; it is unclear whether these impairments take longer than six months to abate or if they are permanent. Benzodiazepines may also cause or worsen depression.[4] Infusions or repeated intravenous injections of diazepam when managing seizures for example may lead to drug toxicity including respiratory depression, sedation as well as hypotension. Tolerance may also develop to infusions of diazepam if it is given for longer than 24 hours.[4] Adverse effects such as sedation, benzodiazepine dependence and abuse potential limit the use of benzodiazepines.[42]
Diazepam has a range of side-effects that are common to most benzodiazepines. Most common side-effects include:
- Suppression of REM sleep
- Impaired motor function
- Impaired coordination
- Impaired balance
- Dizziness and nausea
- Depression[43]
- Reflex tachycardia[44]
Less commonly paradoxical side-effects can occur and include nervousness, irritability, excitement, worsening of seizures, insomnia, muscle cramps, changes in libido (increased or decreased libido) and in some cases, rage, and violence. These adverse reactions are more likely to occur in children, the elderly, individuals with a history of drug or alcohol abuse and people with a history of aggression.[4][45][46][47] Diazepam may increase, in some people, the propensity toward self-harming behaviours and, in extreme cases, may provoke suicidal tendencies or acts.[48] Very rarely dystonia can occur.[49]
Diazepam may impair the ability to drive vehicles or operate machinery. The impairment is worsened by consumption of alcohol, because both act as central nervous system depressants.[28]
During the course of therapy, tolerance to the sedative effects usually develops, but not to the anxiolytic and myorelaxant effects.[50]
Patients with severe attacks of apnea during sleep may suffer respiratory depression (hypoventilation) leading to respiratory arrest and death.
Diazepam in doses of 5 mg or more causes significant deterioration in alertness performance combined with increased feelings of sleepiness.[51]
Tolerance and dependence
Diazepam as with other benzodiazepine drugs can cause tolerance, physical dependence, addiction and what is known as the benzodiazepine withdrawal syndrome. Withdrawal from diazepam or other benzodiazepines often leads to withdrawal symptoms that are similar to those seen during barbiturate or alcohol withdrawal. The higher the dose and the longer the drug is taken the greater the risk of experiencing unpleasant withdrawal symptoms. Withdrawal symptoms can occur from standard dosages and also after short-term use and can range from insomnia and anxiety to more serious symptoms including seizures and psychosis. Withdrawal symptoms can sometimes resemble pre-existing conditions and be misdiagnosed. Diazepam may produce less intense withdrawal symptoms due to its long elimination half-life. Benzodiazepine treatment should be discontinued as soon as possible via a slow and gradual dose reduction regime.[4][52] Tolerance develops to the therapeutic effects of benzodiazepines; for example tolerance occurs to the anticonvulsant effects and as a result benzodiazepines are not generally recommended for the long-term management of epilepsy. Dose increases may overcome the effects of tolerance, however, tolerance may then develop to the higher dose and adverse effects may increase. The mechanism of tolerance to benzodiazepines includes, uncoupling of receptor sites, alterations in gene expression, down regulation of receptor sites and desensitisation of receptor sites to the effect of GABA. Approximately one third of individuals who take benzodiazepines for longer than 4 weeks become dependent and experience a withdrawal syndrome upon cessation.[4] The difference in rates of withdrawal (50–100%) varies depending on the patient sample being investigated. For example, a random sample of long-term benzodiazepine users typically finds that around 50% will experience little or no withdrawal symptoms, with the other 50% experiencing notable withdrawal symptoms. Certain select patient groups will show a higher rate of notable withdrawal symptoms, up to 100%.[53] Rebound anxiety, more severe than baseline anxiety, is also a common withdrawal symptom when discontinuing diazepam or other benzodiazepines.[54] Diazepam is therefore only recommended for short-term therapy at the lowest possible dose owing to risks of severe withdrawal problems from low doses even after gradual reduction.[55] There is a significant risk of pharmacological dependence on diazepam and patients experiencing symptoms of benzodiazepine withdrawal syndrome if it is taken for 6 weeks or longer.[56] In humans tolerance to the anticonvulsant effects of diazepam occurs frequently.[57]
Dependence
Improper or excessive use of Diazepam can lead to psychological dependence/drug addiction.[58] At a particularly high risk for diazepam misuse, abuse or psychological dependence are:
- People with a history of alcohol or drug abuse or dependence[28][59] Diazepam increases craving for alcohol in problem alcohol consumers. Diazepam also increases the volume of alcohol consumed by problem drinkers.[60]
- People with severe personality disorders, such as Borderline Personality Disorder[61]
Patients from the aforementioned groups should be monitored very closely during therapy for signs of abuse and development of dependence. Therapy should be discontinued if any of these signs are noted, although if physical dependence has developed therapy must still be discontinued gradually to avoid severe withdrawal symptoms. Long-term therapy in these people is not recommended.[28][59]
People suspected of being physiologically dependent on benzodiazepine drugs should be very gradually tapered off the drug. Although rare, withdrawals can be life-threatening particularly when excessive doses have been taken for extended periods of time. Equal prudence should be used whether dependence has occurred in therapeutic or recreational contexts.
Diazepam in and of itself is not a recreational drug, but may be used to either enhance or "come down" from the effects of other recreational drugs. For example, diazepam increases the euphoriant effects of heroin (and other recreational opiates), yet decreases the undesirable side-effects of cocaine and/or methamphetamine come-down.
Overdose
Main article: Benzodiazepine overdoseAn individual that has consumed too much diazepam will typically display one or more of the following symptoms in a period of approximately four hours immediately following a suspected overdose:[28][62]
- Drowsiness
- Mental confusion
- Hypotension
- Impaired motor functions
- Impaired reflexes
- Impaired coordination
- Impaired balance
- Dizziness
- Coma
Although not usually fatal when taken alone, a diazepam overdose is considered a medical emergency and generally requires the immediate attention of medical personnel. The antidote for an overdose of diazepam (or any other benzodiazepine) is flumazenil (Anexate). This drug is only used in cases with severe respiratory depression or cardiovascular complications. Because flumazenil is a short-acting drug, and the effects of diazepam can last for days, several doses of flumazenil may be necessary. Artificial respiration and stabilization of cardiovascular functions may also be necessary. Although not routinely indicated, activated charcoal can be used for decontamination of the stomach following a diazepam overdose. Emesis is contraindicated. Dialysis is minimally effective. Hypotension may be treated with levarterenol or metaraminol.[12][28][62][63]
The oral LD50 (lethal dose in 50% of the population) of diazepam is 720 mg/kg in mice and 1240 mg/kg in rats.[28] D. J. Greenblatt and colleagues reported in 1978 on two patients who had taken 500 and 2000 mg of diazepam, respectively, went into moderately deep comas, and were discharged within 48 hours without having experienced any important complications, in spite of having high concentrations of diazepam and its metabolites, esmethyldiazepam, oxazepam, and temazepam; according to samples taken in the hospital and as follow-up.[64]
Overdoses of diazepam with alcohol, opiates and/or other depressants may be fatal.[63][65]
An Australian study has found people who take sleeping pills or anti-anxiety medications are more dangerous on the roads than drunk drivers.[66]
Interactions
If diazepam is to be administered concomitantly with other drugs, attention should be paid to the possible pharmacological interactions. Particular care should be taken with drugs that enhance the effects of diazepam, such as barbiturates, phenothiazines, narcotics and antidepressants.[28]
Diazepam does not increase or decrease hepatic enzyme activity, and does not alter the metabolism of other compounds. There is no evidence that would suggest diazepam alters its own metabolism with chronic administration.[12]
Agents that have an effect on hepatic cytochrome P450 pathways or conjugation can alter the rate of diazepam metabolism. These interactions would be expected to be most significant with long-term diazepam therapy, and their clinical significance is variable.[12]
- Diazepam increases the central depressive effects of alcohol, other hypnotics/sedatives (e.g., barbiturates), narcotics, other muscle relaxants, certain antidepressants, sedative antihistamines, opiates, and antipsychotics as well as anticonvulsants such as phenobarbital, phenytoin and carbamazepine. The euphoriant effects of opioids may be increased, leading to increased risk of psychological dependence.[4][36][67]
- Cimetidine, omeprazole, oxcarbazepine, ticlopidine, topiramate, ketoconazole, itraconazole, disulfiram, fluvoxamine, isoniazid, erythromycin, probenecid, propranolol, imipramine, ciprofloxacin, fluoxetine and valproic acid prolong the action of diazepam by inhibiting its elimination.[4][12][31]
- Alcohol (ethanol) in combination with diazepam may cause a synergistic enhancement of the hypotensive properties of benzodiazepines and alcohol.[68]
- Oral contraceptives ("the pill") significantly decrease the elimination of desmethyldiazepam, a major metabolite of diazepam.[36][69]
- Rifampin, phenytoin, carbamazepine and phenobarbital increase the metabolism of diazepam, thus decreasing drug levels and effects.[12] Dexamethasone and St John's wort also increase the metabolism of diazepam.[4]
- Diazepam increases the serum levels of phenobarbital.[70]
- Nefazodone can cause increased blood levels of benzodiazepines.[36]
- Cisapride may enhance the absorption, and therefore the sedative activity, of diazepam.[71]
- Small doses of theophylline may inhibit the action of diazepam.[72]
- Diazepam may block the action of levodopa (used in the treatment of Parkinson's Disease).[67]
- Diazepam may alter digoxin serum concentrations.[12]
- Other drugs that may have interactions with diazepam include: Antipsychotics (e.g. chlorpromazine), MAO inhibitors, ranitidine.[36]
- Caffeine may antagonise the effects of diazepam and vice versa.[73]
- Smoking tobacco can enhance the elimination of diazepam and decrease its action.[67]
- Because it acts on the GABA receptor the herb Valerian may produce an adverse effect.[74]
- Foods that acidify the urine can lead to faster absorption and elimination of diazepam, reducing drug levels and activity.[67]
- Foods that alkalinize the urine can lead to slower absorption and elimination of diazepam, increasing drug levels and activity.[12]
- There are conflicting reports as to whether food in general has any effects on the absorption and activity of orally administered diazepam.[67]
Pharmacology
Diazepam is a long acting "classical" benzodiazepine. Other classical benzodiazepines include chlordiazepoxide, clonazepam, lorazepam, oxazepam, nitrazepam, temazepam, flurazepam[citation needed], bromazepam[citation needed], and clorazepate[citation needed].[75] Diazepam has anticonvulsant properties.[76] Diazepam has no effect on GABA levels and no effect on glutamate decarboxylase activity but has a slight effect on gamma-aminobutyric acid transaminase activity. It differs insofar from some other anticonvulsive drugs it was compared with.[77] Benzodiazepines act via micromolar benzodiazepine binding sites as Ca2+ channel blockers and significantly inhibit depolarization-sensitive Calcium uptake in rat nerve cell preparations.[78]
Diazepam inhibits acetylcholine release in mouse hippocampal synaptosomes. This has been found by measuring sodium-dependent high affinity choline uptake in mouse brain cells in vitro, after pretreatment of the mice with diazepam in vivo. This may play a role in explaining diazepam's anticonvulsant properties.[79]
Diazepam binds with high affinity to glial cells in animal cell cultures.[80] Diazepam at high doses has been found to decrease histamine turnover in mouse brain via diazepam's action at the benzodiazepine-GABA receptor complex.[81] Diazepam also decreases prolactin release in rats.[82]
Mechanism of action
See also: BenzodiazepineDiazepam is a benzodiazepine that binds to a specific subunit on the GABAA receptor at a site that is distinct from the binding site of the endogenous GABA molecule. The GABAA receptor is an inhibitory channel which, when activated, decreases neuronal activity. Benzodiazepines do not supplement for the neurotransmitter GABA, rather benzodiazepines such as diazepam bind to a different location on the GABAA receptor with the result that the effects of GABA are enhanced. Benzodiazepines cause an increased opening of the chloride ion channel when GABA binds to its site on the GABAA receptor leading to more chloride ions entering the neuron which in turn leads to enhanced central nervous system depressant effects.[4] Diazepam binds non-selectively to alpha1, alpha2, alpha3 and alpha5 subunit containing GABAA receptors.[83]
Because of the role of diazepam as a positive allosteric modulator of GABA, when it binds to benzodiazepine receptors it causes inhibitory effects. This arises from the hyperpolarization of the post-synaptic membrane, owing to the control exerted over negative chloride ions by GABAA receptors.[28][84]
Diazepam appears to act on areas of the limbic system, thalamus, and hypothalamus, inducing anxiolytic effects. Its actions are due to the enhancement of GABA activity.[1][84] Benzodiazepine drugs including diazepam increase the inhibitory processes in the cerebral cortex.[85]
The anticonvulsant properties of diazepam and other benzodiazepines may be in part or entirely due to binding to voltage-dependent sodium channels rather than benzodiazepine receptors. Sustained repetitive firing seems to be limited by benzodiazepines' effect of slowing recovery of sodium channels from inactivation.[86]
The muscle relaxant properties of Diazepam are produced via inhibition of polysynaptic pathways in the spinal cord.[87]
Pharmacokinetics
Generic pack of 5mg Diazepam.
Diazepam can be administered orally, intravenously (needs to be diluted, as it is painful and damaging to veins), intramuscularly (see below), or as a suppository.[12]
When Diazepam is administered orally, it is rapidly absorbed and has a fast onset of action. The onset of action is 1–5 minutes for IV administration and 15–30 minutes for IM administration. The duration of diazepam's peak pharmacological effects is 15 minutes to 1 hour for both routes of administration.[44] The bioavailability after oral administration is 100 percent, and 90 percent after rectal administration. Peak plasma levels occur between 30 minutes and 90 minutes after oral administration and between 30 minutes and 60 minutes after intramuscular administration; after rectal administration peak plasma levels occur after 10 minutes to 45 minutes. Diazepam is highly protein bound with 96 to 99 percent of the absorbed drug being protein bound. The distribution half-life of diazepam is 2 minutes to 13 minutes.[4]
When Diazepam is administered as an intramuscular injection, absorption is slow, erratic and incomplete.[8]
Diazepam is highly lipid-soluble, and is widely distributed throughout the body after administration. It easily crosses both the blood-brain barrier and the placenta, and is excreted into breast milk. After absorption, diazepam is redistributed into muscle and adipose tissue. Continual daily doses of diazepam will quickly build up to a high concentration in the body (mainly in adipose tissue), which will be far in excess of the actual dose for any given day.[4][12]
There is preferential storage of Diazepam in some organs including the heart. Absorption by any administered route and the risk of accumulation is significantly increased in the neonate and there is clinical justification to recommend the withdrawal of diazepam during pregnancy and breast feeding.[88]
Diazepam undergoes oxidative metabolism by Demethylation (CYP 2C9, 2C19, 2B6, 3A4, and 3A5), hydroxylation (CYP 3A4 and 2C19) as well as glucuronidation in the liver as part of the cytochrome P450 enzyme system. Diazepam has several pharmacologically active metabolites. The main active metabolite of diazepam is desmethyldiazepam (also known as nordazepam or nordiazepam). Diazepam's other active metabolites include the minor active metabolites temazepam and oxazepam. These metabolites are conjugated with glucuronide, and are excreted primarily in the urine. Because of these active metabolites, the serum values of diazepam alone are not useful in predicting the effects of the drug. Diazepam has a biphasic half-life of about 1–3 and 2–7 days for the active metabolite desmethyldiazepam.[4]
Most of the drug is metabolised; very little diazepam is excreted unchanged.[12]
The elimination half-life of diazepam and also the active metabolite desmethyldiazepam increases significantly in the elderly, which may result in prolonged action as well as accumulation of the drug during repeated administration.[89]
Detection in body fluids
Diazepam may be quantitated in blood or plasma to confirm a diagnosis of poisoning in hospitalized patients, provide evidence in an impaired driving arrest or to assist in a medicolegal death investigation. Blood or plasma diazepam concentrations are usually in a range of 0.1-1.0 mg/L in persons receiving the drug therapeutically, 1–5 mg/L in those arrested for impaired driving and 2–20 mg/L in victims of acute overdosage. Most commercial immunoassays for the benzodiazepine class of drugs will cross-react with diazepam, but confirmation and quantitation is usually performed using chromatographic techniques.[90][91][92]
Physical properties
Diazepam occurs as solid white or yellow crystals and has a melting point of 131.5 to 134.5 °C. It is odorless, and has a slightly bitter taste. The British Pharmacopoeia lists diazepam as being very slightly soluble in water, soluble in alcohol and freely soluble in chloroform. The United States Pharmacopoeia lists diazepam as soluble 1 in 16 of ethyl alcohol, 1 in 2 of chloroform, 1 in 39 of ether, and practically insoluble in water. The pH of diazepam is neutral (i.e., pH = 7). Diazepam has a shelf-life of 5 years for oral tablets and 3 years for IV/IM solution.[12] Diazepam should be stored at room temperature (15–30°C). The solution for parenteral injection should be protected from light and kept from freezing. The oral forms should be stored in air-tight containers and protected from light.[31]
Diazepam can absorb into plastic, and, therefore, diazepam solution is not stored in plastic bottles or syringes, etc. It can absorb into plastic bags and tubing used for intravenous infusions. Absorption appears to be dependent on several factors such as temperature, concentration, flow rates, and tube length. Diazepam should not be administered if a precipitate has formed and will not dissolve.[31]
Chemistry
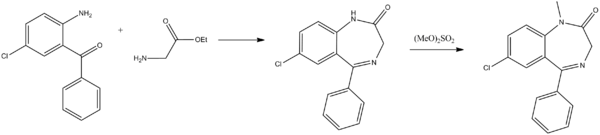
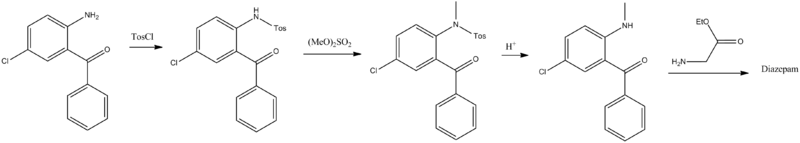
From a chemical point of view, diazepam, 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one, is the most simple of all of the examined derivatives of 1,4-benzodiazepin-2-ones. Various ways for the synthesis of diazepam from 2-amino-5-chlorobenzophenone have been proposed. The first two ways consist of the direct cyclocondensation of 2-amino-5-chlorobenzophenone or 2-methylamino-5-chlorobenzophenone with the ethyl ester of glycine hydrochloride. The amide nitrogen atom of the obtained 7-chloro-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepin-2-one, is methylated by dimethylsulfate, which leads to the formation of diazepam.
The second way differs from the first in that the methylation of nitrogen is accomplished before the cyclocondensation reaction. In order to do this, the initial 2-amino-5-chlorobenzophenone is first tosylated by p-toluenesulfonylchloride and the obtained tosylate transformed into the N-sodium salt, which is then alkylated by dimethylsulfate. The resulting 2-N-tosyl-N-methyl-5-chlorobenzophenone is hydrolyzed in an acidic medium, giving 2-methylamino-5-chlorobenzophenone, which undergoes cyclocondensation by reaction with ethyl ester of glycine hydrochloride, forming the desired diazepam.[93][94][95][96][97]
History
Diazepam was the second benzodiazepine to be invented by Dr. Leo Sternbach of Hoffmann-La Roche, following chlordiazepoxide (Librium) which was approved for use in 1960. Released in 1963 as an improved version of Librium, diazepam became incredibly popular, helping Roche to become a pharmaceutical industry giant. It is two and a half times more potent than its predecessor, which it quickly surpassed in terms of sales. After this initial success, other pharmaceutical companies began to introduce other benzodiazepine derivatives.[98]
The benzodiazepines gained popularity among medical professionals as an improvement upon barbiturates, which have a comparatively narrow therapeutic index, and are far more sedating at therapeutic doses. The benzodiazepines are also far less dangerous; death rarely results from diazepam overdose, except in cases where it is consumed with large amounts of other depressants (such as alcohol or other sedatives).[63] Benzodiazepine drugs such as diazepam initially had widespread public support, but with time the view changed to one of growing criticism and calls for restrictions on their prescription.[99]
Diazepam was the top-selling pharmaceutical in the United States from 1969 to 1982, with peak sales in 1978 of 2.3 billion tablets.[98] Diazepam, along with oxazepam, nitrazepam and temazepam, represents 82% of the benzodiazepine market in Australia.[100] While psychiatrists continue to prescribe diazepam for the short-term relief of anxiety, neurology has taken the lead in prescribing diazepam for the palliative treatment of certain types of epilepsy and spastic activity, for example, forms of paresis. It is also the first line of defense for a rare disorder called stiff-person syndrome.[27] In recent years, the public perception of benzodiazepines has become increasingly negative.[83]
Society and culture
Recreational use
See also: Benzodiazepine drug misuseDiazepam is a drug of potential abuse and can cause serious problems of addiction and as a result is scheduled. Urgent action by national governments has been recommended to improve prescribing patterns of benzodiazepines such as diazepam.[5][83] A single dose of diazepam modulates the dopamine system in similar ways to how morphine and alcohol modulate the dopaminergic pathways.[101] Between 50 and 64% of rats will self administer diazepam.[102] Benzodiazepines including diazepam in animal studies have been shown to increase reward seeking behaviours by increasing impulsivity, which may suggest an increased risk of addictive behavioural patterns with usage of diazepam or other benzodiazepines.[103] In addition diazepam has been shown to be able to substitute for the behavioural effects of barbiturates in a primate study.[104] Diazepam has been found as an adulterant in heroin.[105]
Diazepam drug misuse can occur either through recreational misuse where the drug is taken to achieve a high or when the drug is continued long term against medical advice.[106]
Sometimes Diazepam is used by stimulant users to "come down" and sleep and to help control the urge to binge.[107]
A large-scale nationwide USA government study conducted by SAMHSA found that benzodiazepines in the USA are the most frequently abused pharmaceutical with 35% of drug-related visits to the Emergency Department involved benzodiazepines. Benzodiazepines are more commonly abused than opiate pharmaceuticals, which accounted for 32% of visits to the emergency department. No other pharmaceutical is more commonly abused than benzodiazepines. Males abuse benzodiazepines as commonly as females. Of drugs used in attempted suicide benzodiazepines are the most commonly used pharmaceutical drug, with 26% of attempted suicides involving benzodiazepines. The most commonly abused benzodiazepine is, however, alprazolam. Clonazepam is the second-most-abused benzodiazepine. Lorazepam is the third-most-abused benzodiazepine, and diazepam the fourth-most-abused benzodiazepine in the USA.[108]
Benzodiazepines, including Diazepam, nitrazepam, and flunitrazepam account for the largest volume of forged drug prescriptions in Sweden, a total of 52% of drug forgeries being for benzodiazepines.[109]
Diazepam was detected in 26% of cases of people suspected of driving under the influence of drugs in Sweden and its active metabolite nordazepam was detected in 28% of cases. Other benzodiazepines and zolpidem and zopiclone also were found in high numbers. Many drivers had blood levels far exceeding the therapeutic dose range suggesting a high degree of abuse potential for benzodiazepines and zolpidem and zopiclone.[110] In Northern Ireland in cases where drugs were detected in samples from impaired drivers who were not impaired by alcohol, benzodiazepines were found to be present in 87% of cases. Diazepam was the most commonly detected benzodiazepine.[111]
Legal status
Diazepam is regulated in most countries as a prescription drug:
- International: Diazepam is a Schedule IV controlled drug under the Convention on Psychotropic Substances.[112]
- UK: classified as a controlled drug, listed under Schedule IV, Part I (CD Benz POM) of the Misuse of Drugs Regulations 2001, allowing possession with a valid prescription. The Misuse of Drugs Act 1971 makes it illegal to possess the drug without a prescription, and for such purposes it is classified as a Class C drug. "List of Controlled Drugs". http://www.homeoffice.gov.uk/documents/cdlist2835.pdf?view=Binary.
- Germany: classified as a prescription drug, or in high dosage as a restricted drug (Betäubungsmittelgesetz, Anhang III).[113]
Judicial executions
The State of California offers diazepam to condemned inmates as a pre-execution sedative as part of their Lethal Injection program.[114]
Veterinary uses
Diazepam is used as a short-term sedative and anxiolytic for cats and dogs. It is also used for short-term treatment of seizures in dogs and short-term and long-term treatment of seizures in cats. It can also be used as an appetite stimulant.[115][116] For emergent treatment of seizures, the typical dose is 0.5cmg/kg intravenously, or 1–2c;mg/kg of the injectable solution administered in the rectum.[117]
References
- ^ a b "Diazepam". PubChem. National Institute of Health: National Library of Medicine. 2006. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=3016. Retrieved 2006-03-11.
- ^ "Diazepam". Medical Subject Headings (MeSH). National Library of Medicine. 2006. http://www.nlm.nih.gov/cgi/mesh/2006/MB_cgi?mode=&term=Diazepam. Retrieved 2006-03-10.
- ^ Mandrioli, R., L. Mercolini, M.A. Raggi (October 2008). "Benzodiazepine metabolism: an analytical perspective". Curr. Drug Metab. 9 (8): 827–44. doi:10.2174/138920008786049258. PMID 18855614. http://www.benthamdirect.org/pages/content.php?CDM/2008/00000009/00000008/0009F.SGM.
- ^ a b c d e f g h i j k l m n o p q r s t Riss, J.; Cloyd, J.; Gates, J.; Collins, S. (Aug 2008). "Benzodiazepines in epilepsy: pharmacology and pharmacokinetics" (PDF). Acta Neurol Scand 118 (2): 69–86. doi:10.1111/j.1600-0404.2008.01004.x. PMID 18384456. http://www3.interscience.wiley.com/cgi-bin/fulltext/120119477/PDFSTART.
- ^ a b Dièye, AM.; Sylla, M.; Ndiaye, A.; Ndiaye, M.; Sy, GY.; Faye, B. (Jun 2006). "Benzodiazepines prescription in Dakar: a study about prescribing habits and knowledge in general practitioners, neurologists and psychiatrists". Fundam Clin Pharmacol 20 (3): 235–8. doi:10.1111/j.1472-8206.2006.00400.x. PMID 16671957.
- ^ "WHO Model List of Essential Medicines" (PDF). World Health Organization. March 2005. http://whqlibdoc.who.int/hq/2005/a87017_eng.pdf. Retrieved 2006-03-12.
- ^ L. H. Sternbach, E. Reeder, O. Keller, W. Metlesics (1961). "Quinazolines and 1,4-benzodiazepines III substituted 2-amino-5-phenyl-3H-1,4-benzodiazepine 4-oxides". J. Org. Chem. 26 (11): 4488–4497. doi:10.1021/jo01069a069.
- ^ a b c d e Drug Bank - Diazepam. http://redpoll.pharmacy.ualberta.ca/drugbank/cgi-bin/getCard.cgi?CARD=APRD00642.txt.
- ^ Bråthen, G.; Ben-Menachem, E.; Brodtkorb, E.; Galvin, R.; Garcia-Monco, JC.; Halasz, P.; Hillbom, M.; Leone, MA. et al. (Aug 2005). "EFNS guideline on the diagnosis and management of alcohol-related seizures: report of an EFNS task force". Eur J Neurol 12 (8): 575–81. doi:10.1111/j.1468-1331.2005.01247.x. PMID 16053464.
- ^ Walker, M. (Sep 2005). "Status epilepticus: an evidence based guide". BMJ 331 (7518): 673–7. doi:10.1136/bmj.331.7518.673. PMC 1226249. PMID 16179702. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1226249.
- ^ Prasad, K.; Al-Roomi, K.; Krishnan, PR.; Sequeira, R.; Prasad, Kameshwar (October 2005). Prasad, Kameshwar. ed. "Anticonvulsant therapy for status epilepticus" (PDF). Cochrane Database Syst Rev (4): CD003723. doi:10.1002/14651858.CD003723.pub2. PMID 16235337. http://mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD003723/pdf_fs.html.
- ^ a b c d e f g h i j k l m n o p Pere Munne/M. Ruse, Ed. (1990/1998 Ed.). "Diazepam". Inchem.org. Inchem.org. http://www.inchem.org/documents/pims/pharm/pim181.htm. Retrieved 2006-03-11.
- ^ Isojärvi, JI; Tokola RA. (December 1998). "Benzodiazepines in the treatment of epilepsy in people with intellectual disability". J Intellect Disabil Res. 42 (1): 80–92. PMID 10030438.
- ^ Kaplan, PW. (Nov 2004). "Neurologic aspects of eclampsia". Neurol Clin 22 (4): 841–61. doi:10.1016/j.ncl.2004.07.005. PMID 15474770.
- ^ Duley, L. (Feb 2005). "Evidence and practice: the magnesium sulphate story". Best Pract Res Clin Obstet Gynaecol 19 (1): 57–74. doi:10.1016/j.bpobgyn.2004.10.010. PMID 15749066.
- ^ Zeilhofer, HU.; Witschi, R.; Hösl, K. (May 2009). "Subtype-selective GABAA receptor mimetics--novel antihyperalgesic agents?" (PDF). J Mol Med 87 (5): 465–9. doi:10.1007/s00109-009-0454-3. PMID 19259638. http://www.springerlink.com/content/u3555g26k736101p/fulltext.pdf.
- ^ Mezaki T, Hayashi A, Nakase H, Hasegawa K (September 2005). "[Therapy of dystonia in Japan]" (in Japanese). Rinsho Shinkeigaku 45 (9): 634–42. PMID 16248394.
- ^ Kachi T (December 2001). "[Medical treatment of dystonia]" (in Japanese). Rinsho Shinkeigaku 41 (12): 1181–2. PMID 12235832.
- ^ Ashton H (2005). "The diagnosis and management of benzodiazepine dependence" (PDF). Curr Opin Psychiatry 18 (3): 249–55. doi:10.1097/01.yco.0000165594.60434.84. PMID 16639148. http://www.benzo.org.uk/amisc/ashdiag.pdf.
- ^ Mañon-Espaillat R, Mandel S (1999). "Diagnostic algorithms for neuromuscular diseases". Clin Podiatr Med Surg 16 (1): 67–79. PMID 9929772.
- ^ Kamen, L.; Henney, HR.; Runyan, JD. (Feb 2008). "A practical overview of tizanidine use for spasticity secondary to multiple sclerosis, stroke, and spinal cord injury". Curr Med Res Opin 24 (2): 425–39. doi:10.1185/030079908X261113. PMID 18167175.
- ^ Bajgar, J. (2004). "Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment". Adv Clin Chem. Advances in Clinical Chemistry 38: 151–216. doi:10.1016/S0065-2423(04)38006-6. ISBN 978-0-12-010338-6. PMID 15521192.
- ^ Karande, S. (Mar 2007). "Febrile seizures: a review for family physicians". Indian J Med Sci 61 (3): 161–72. doi:10.4103/0019-5359.30753. PMID 17337819. http://www.indianjmedsci.org/article.asp?issn=0019-5359;year=2007;volume=61;issue=3;spage=161;epage=172;aulast=Karande.
- ^ Cesarani A, Alpini D, Monti B, Raponi G (March 2004). "The treatment of acute vertigo". Neurol. Sci. 25 Suppl 1: S26–30. doi:10.1007/s10072-004-0213-8. PMID 15045617.
- ^ Lader M, Tylee A, Donoghue J (2009). "Withdrawing benzodiazepines in primary care". CNS Drugs 23 (1): 19–34. doi:10.2165/0023210-200923010-00002. PMID 19062773.
- ^ Okoromah, C. N.; F. E. Lesi (2004). Okoromah, Christy AN. ed. "Diazepam for treating tetanus". Cochrane database of systematic reviews (Online) (1): CD003954. doi:10.1002/14651858.CD003954.pub2. PMID 14974046.
- ^ a b c "Diazepam: indications". Rxlist.com. RxList Inc.. January 24, 2005. http://www.rxlist.com/cgi/generic/diazepam_ids.htm. Retrieved 2006-03-11.
- ^ a b c d e f g h i j k Thomson Healthcare (Micromedex) (March 2000). "Diazepam". Prescription Drug Information. Drugs.com. http://www.drugs.com/pdr/diazepam.html. Retrieved 2006-03-11.
- ^ Hyperbaric Medicine Practice, Second Edition. Best Publishing Company. 1999. ISBN 0-941332-78-0.
- ^ "International AED Database". ILAE. http://www.ilae.org/Visitors/Centre/AEDs/index.cfm. Retrieved 2009-09-16.
- ^ a b c d Mikota, Susan K. and Plumb, Donald C. (2005). "Diazepam". The Elephant Formulary. Elephant Care International. http://www.elephantcare.org/Drugs/diazepam.htm.
- ^ Pharmaceutical Patents. http://www.pharmcast.com/Patents100/Yr2004/Oct2004/101904/6805853_Diazepam101904.htm
- ^ U.S. Army Medical Research Institute of Chemical Defense, Medical Management of Chemical Casualties Handbook, Third Edition (June 2000), Aberdeen Proving Ground, MD, pp. 118–126.
- ^ Epocrates. "Diazepam Contraindications and Cautions". USA: Epocrates Online. https://online.epocrates.com/u/103193/diazepam/Contraindications+Cautions. Retrieved 16 December 2008.
- ^ Authier, N.; Balayssac, D.; Sautereau, M.; Zangarelli, A.; Courty, P.; Somogyi, AA.; Vennat, B.; Llorca, PM. et al. (November 2009). "Benzodiazepine dependence: focus on withdrawal syndrome". Ann Pharm Fr 67 (6): 408–13. doi:10.1016/j.pharma.2009.07.001. PMID 19900604.
- ^ a b c d e f g "Diazepam". PDRHealth.com. PDRHealth.com. 2006. Archived from the original on 2006-01-17. http://web.archive.org/web/20060117065720/http://www.pdrhealth.com/drug_info/rxdrugprofiles/drugs/val1473.shtml. Retrieved 2006-03-10.
- ^ a b "Diazepam: precautions". Rxlist.com. RxList Inc.. January 24, 2005. http://www.rxlist.com/cgi/generic/diazepam_wcp.htm. Retrieved 2006-03-10.
- ^ Shats V; Kozacov S. (June 1, 1995). "[Falls in the geriatric department: responsibility of the care-giver and the hospital]". Harefuah 128 (11): 690–3. PMID 7557666.
- ^ Kanto JH. (May 1982). "Use of benzodiazepines during pregnancy, labour and lactation, with particular reference to pharmacokinetic considerations". Drugs. 23 (5): 354–80. doi:10.2165/00003495-198223050-00002. PMID 6124415.
- ^ McElhatton PR. (Nov–Dec 1994). "The effects of benzodiazepine use during pregnancy and lactation". Reprod Toxicol. 8 (6): 461–75. doi:10.1016/0890-6238(94)90029-9. PMID 7881198.
- ^ Yudofsky, Stuart C.; Hales, Robert E. (1 December 2007). The American Psychiatric Publishing Textbook of Neuropsychiatry and Behavioral Neurosciences, Fifth Edition (American Psychiatric Press Textbook of Neuropsychiatry). USA: American Psychiatric Publishing, Inc.. pp. 583–584. ISBN 978-1-58562-239-9. http://books.google.co.uk/books?id=f5BEk-6yO_4C&pg=PA583.
- ^ Whiting, PJ. (Feb 2006). "GABA-A receptors: a viable target for novel anxiolytics?". Curr Opin Pharmacol 6 (1): 24–9. doi:10.1016/j.coph.2005.08.005. PMID 16359919.
- ^ Kay DW, Fahy T, Garside RF (December 1970). "A seven-month double-blind trial of amitriptyline and diazepam in ECT-treated depressed patients". Br J Psychiatry 117 (541): 667–71. doi:10.1192/bjp.117.541.667. PMID 4923720. http://bjp.rcpsych.org/cgi/content/abstract/117/541/667.
- ^ a b Langsam, Yedidyah (<!––Unknown––>). "DIAZEPAM (VALIUM AND OTHERS)". Brooklyn College (Eilat.sci.Brooklyn.CUNY.edu). http://eilat.sci.brooklyn.cuny.edu/newnyc/DRUGS/Diazepam.htm. Retrieved 2006-03-23.
- ^ Marrosu, F.; G. Marrosu, M. G. Rachel, G. Biggio (July–September 1987). "Paradoxical reactions elicited by diazepam in children with classic autism". Functional Neurology 2 (3): 355–361. PMID 2826308.
- ^ "Diazepam: Side Effects". RxList.com. http://www.rxlist.com/cgi/generic/diazepam_ad.htm. Retrieved September 26, 2006.
- ^ Michel, L.; J. P. Lang (November–December 2003). "Benzodiazépines et passage à l'acte criminel / Benzodiazepines and forensic aspects". L'Encéphale 29 (6): 479–85. PMID 15029082. http://www.masson.fr/masson/portal/bookmark?Global=1&Page=18&MenuIdSelected=106&MenuItemSelected=0&MenuSupportSelected=0&CodeProduct4=539&CodeRevue4=ENC&Path=REVUE/ENC/2003/29/6/ARTICLE11106200473.xml&Locations=.
- ^ Berman ME, Jones GD, McCloskey MS (February 2005). "The effects of diazepam on human self-aggressive behavior". Psychopharmacology (Berl.) 178 (1): 100–6. doi:10.1007/s00213-004-1966-8. PMID 15316710.
- ^ Pérez Trullen JM, Modrego Pardo PJ, Vázquez André M, López Lozano JJ (1992). "Bromazepam-induced dystonia". Biomed. Pharmacother. 46 (8): 375–6. doi:10.1016/0753-3322(92)90306-R. PMID 1292648.
- ^ Hriscu, A.; F. Gherase, V. Nastasa, and E. Hriscu (October–December 2002). "[An experimental study of tolerance to benzodiazepines]". Revista Medico-Chirurgicală̆ a Societă̆ţ̜ii de Medici ş̧i Naturaliş̧ti din Iaş̧i 106 (4): 806–811. PMID 14974234.
- ^ Kozená L; Frantik E, Horváth M. (May 1995). "Vigilance impairment after a single dose of benzodiazepines". Psychopharmacology (Berl). 119 (1): 39–45. doi:10.1007/BF02246052. PMID 7675948.
- ^ MacKinnon GL; Parker WA. (1982). "Benzodiazepine withdrawal syndrome: a literature review and evaluation". The American journal of drug and alcohol abuse. 9 (1): 19–33. doi:10.3109/00952998209002608. PMID 6133446.
- ^ Onyett SR (April 1989). "The benzodiazepine withdrawal syndrome and its management". The Journal of the Royal College of General Practitioners 39 (321): 160–3. PMC 1711840. PMID 2576073. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1711840.
- ^ Chouinard G; Labonte A, Fontaine R, Annable L (1983). "New concepts in benzodiazepine therapy: rebound anxiety and new indications for the more potent benzodiazepines". Prog Neuropsychopharmacol Biol Psychiatry 7 (4–6): 669–73. doi:10.1016/0278-5846(83)90043-X. PMID 6141609.
- ^ Lader M. (December 1987). "Long-term anxiolytic therapy: the issue of drug withdrawal". The Journal of clinical psychiatry. 48: 12–6. PMID 2891684.
- ^ Murphy SM, Owen R, Tyrer P. (1989). "Comparative assessment of efficacy and withdrawal symptoms after 6 and 12 weeks' treatment with diazepam or buspirone". The British Journal of Psychiatry: the journal of mental science. 154 (4): 529–34. doi:10.1192/bjp.154.4.529. PMID 2686797.
- ^ Loiseau P (1983). "[Benzodiazepines in the treatment of epilepsy]". Encephale 9 (4 Suppl 2): 287B–292B. PMID 6373234.
- ^ "Treating Anxiety -- Avoiding Dependence on Xanax, Klonopin, Valium, and Other Antianxiety Drugs". johnshopkinshealthalerts.com. Johnshopkinshealthalerts.com. 2005. http://www.johnshopkinshealthalerts.com/reports/depression_anxiety/59-1.html?type=pf. Retrieved 2007-12-23.
- ^ a b "Diazepam: abuse and dependence". Rxlist.com. RxList Inc.. January 24, 2005. http://www.rxlist.com/cgi/generic/diazepam_ad.htm#DA. Retrieved 2006-03-10.
- ^ Poulos CX, Zack M (November 2004). "Low-dose diazepam primes motivation for alcohol and alcohol-related semantic networks in problem drinkers". Behav Pharmacol 15 (7): 503–12. doi:10.1097/00008877-200411000-00006. PMID 15472572.
- ^ Vorma, Helena; Hannu H. Naukkarinen, Seppo J. Sarna, and Kimmo I. Kuoppasalmi (2005). "Predictors of Benzodiazepine Discontinuation in Subjects Manifesting Complicated Dependence" (PDF). Substance Use & Misuse 40 (4): 499–510. doi:10.1081/JA-200052433. PMID 15830732.
- ^ a b "Diazepam: overdose". Rxlist.com. RxList Inc.. January 24, 2005. http://www.rxlist.com/cgi/generic/diazepam_od.htm. Retrieved 2006-03-10.
- ^ a b c Barondes, Samuel H. (2003). Better Than Prozac. New York: Oxford University Press. pp. 47–59. ISBN 0-19-515130-5.
- ^ Greenblatt, D. J.; E. Woo, M. D. Allen, P. J. Orsulak, and R. I. Shader (October 20, 1978). "Rapid recovery from massive diazepam overdose". Journal of the American Medical Association 240 (17): 1872–4. doi:10.1001/jama.240.17.1872. PMID 357765.
- ^ Lai, SH; Yao YJ, Lo DS. (October 2006). "A survey of buprenorphine related deaths in Singapore". Forensic Sci Int. 162(1–3) (1–3): 80–6. doi:10.1016/j.forsciint.2006.03.037. PMID 16879940.
- ^ "Valium users worse drivers than drunks". Sydney Morning Herald. 20 October 2010. http://www.smh.com.au/lifestyle/wellbeing/valium-users-worse-drivers-than-drunks-20101019-16sl7.html. Retrieved 20 October 2010.
- ^ a b c d e Holt, Gary A. (1998). Food and Drug Interactions: A Guide for Consumers. Chicago: Precept Press. pp. 90–91. ISBN 0-944496-59-8.
- ^ Zácková P; Kvĕtina J, Nĕmec J, Nĕmcová J. (December 1982). "Cardiovascular effects of diazepam and nitrazepam in combination with ethanol". Pharmazie. 37 (12): 853–6. PMID 7163374.
- ^ Back DJ; Orme ML. (June 1990). "Pharmacokinetic drug interactions with oral contraceptives". Clin Pharmacokinet. 18 (6): 472–84. doi:10.2165/00003088-199018060-00004. PMID 2191822.
- ^ Bendarzewska-Nawrocka B; Pietruszewska E, Stepień L, Bidziński J, Bacia T. (Jan-Feb 1980). "[Relationship between blood serum luminal and diphenylhydantoin level and the results of treatment and other clinical data in drug-resistant epilepsy]". Neurol Neurochir Pol. 14 (1): 39–45. PMID 7374896.
- ^ Bateman, D.N. (1986). "The action of cisapride on gastric emptying and the pharmacodynamics and pharmacokinetics of oral diazepam". Eur J Clin Pharmacol. 30 (2): 205–8. doi:10.1007/BF00614304. PMID 3709647.
- ^ Mattila, M. J.; E. Nuotto (1983). "Caffeine and theophylline counteract diazepam effects in man". Medical Biology 61 (6): 337–343. PMID 6374311.
- ^ Mattila, Me; Mattila, Mj; Nuotto, E (Apr 1992). "Caffeine moderately antagonizes the effects of triazolam and zopiclone on the psychomotor performance of healthy subjects". Pharmacology & toxicology 70 (4): 286–9. doi:10.1111/j.1600-0773.1992.tb00473.x. ISSN 0901-9928. PMID 1351673.
- ^ Possible Interactions with: Valerian, University of Maryland Medical Center, http://www.umm.edu/altmed/articles/valerian-000934.htm
- ^ Braestrup C; Squires RF. (1 April 1978). "Pharmacological characterization of benzodiazepine receptors in the brain". Eur J Pharmacol 48 (3): 263–70. doi:10.1016/0014-2999(78)90085-7. PMID 639854.
- ^ Chweh AY; Swinyard EA, Wolf HH, Kupferberg HJ (February 25, 1985). "Effect of GABA agonists on the neurotoxicity and anticonvulsant activity of benzodiazepines". Life Sci 36 (8): 737–44. doi:10.1016/0024-3205(85)90193-6. PMID 2983169.
- ^ Battistin L; Varotto M, Berlese G, Roman G (February 1984). "Effects of some anticonvulsant drugs on brain GABA level and GAD and GABA-T activities". Neurochem Res 9 (2): 225–31. doi:10.1007/BF00964170. PMID 6429560.
- ^ Taft WC; DeLorenzo RJ (May 1984). "Micromolar-affinity benzodiazepine receptors regulate voltage-sensitive calcium channels in nerve terminal preparations" (PDF). Proc Natl Acad Sci USA 81 (10): 3118–22. doi:10.1073/pnas.81.10.3118. PMC 345232. PMID 6328498. http://www.pnas.org/cgi/reprint/81/10/3118.pdf.
- ^ Miller JA, Richter JA (January 1985). "Effects of anticonvulsants in vivo on high affinity choline uptake in vitro in mouse hippocampal synaptosomes". British Journal of Pharmacology 84 (1): 19–25. PMC 1987204. PMID 3978310. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1987204.
- ^ Gallager DW, Mallorga P, Oertel W, Henneberry R, Tallman J (February 1981). "[3HDiazepam binding in mammalian central nervous system: a pharmacological characterization"]. The Journal of Neuroscience 1 (2): 218–25. PMID 6267221. http://www.jneurosci.org/cgi/pmidlookup?view=long&pmid=6267221.
- ^ Oishi R; Nishibori M, Itoh Y, Saeki K. (May 27, 1986). "Diazepam-induced decrease in histamine turnover in mouse brain". Eur J Pharmacol. 124 (3): 337–42. doi:10.1016/0014-2999(86)90236-0. PMID 3089825.
- ^ Grandison L (1982). "Suppression of prolactin secretion by benzodiazepines in vivo". Neuroendocrinology 34 (5): 369–73. doi:10.1159/000123330. PMID 6979001.
- ^ a b c Atack, JR. (May 2005). "The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics". Expert Opin Investig Drugs 14 (5): 601–18. doi:10.1517/13543784.14.5.601. PMID 15926867.
- ^ a b Barondes, Samuel H. (MONTH 1999). Molecules and Mental Illness. New York: Scientific American Library. pp. 190–194. ISBN 0-7167-6033-9.
- ^ Zakusov VV; Ostrovskaya RU, Kozhechkin SN, Markovich VV, Molodavkin GM, Voronina TA. (October 1977). "Further evidence for GABA-ergic mechanisms in the action of benzodiazepines". Archives internationales de pharmacodynamie et de therapie 229 (2): 313–26. PMID 23084.
- ^ McLean MJ; Macdonald RL. (February 1988). "Benzodiazepines, but not beta carbolines, limit high frequency repetitive firing of action potentials of spinal cord neurons in cell culture". J Pharmacol Exp Ther. 244 (2): 789–95. PMID 2450203.
- ^ Date SK; Hemavathi KG, Gulati OD. (November 1984). "Investigation of the muscle relaxant activity of nitrazepam". Arch Int Pharmacodyn Ther. 272 (1): 129–39. PMID 6517646.
- ^ Olive G; Dreux C. (January 1977). "Pharmacologic bases of use of benzodiazepines in peréinatal medicine". Arch Fr Pediatr. 34 (1): 74–89. PMID 851373.
- ^ Vozeh S. (November 21, 1981). "[Pharmacokinetic of benzodiazepines in old age]". Schweiz Med Wochenschr. 111 (47): 1789–93. PMID 6118950.
- ^ Jones, A. W.; Holmgren, A.; Kugelberg, F. C. (2007). "Concentrations of scheduled prescription drugs in blood of impaired drivers: considerations for interpreting the results". Ther. Drug Monit. 29 (2): 248–260. doi:10.1097/FTD.0b013e31803d3c04. PMID 17417081.
- ^ Fraser, A. D.; Bryan, W. (1991). "Evaluation of the Abbott ADx and TDx serum benzodiazepine immunoassays". J. Anal. Toxicol. 15 (2): 63–65. PMID 1675703.
- ^ Baselt, R. (2011). Disposition of Toxic Drugs and Chemicals in Man (9th ed.). Seal Beach, CA: Biomedical Publications. pp. 471–473. ISBN 978-0-9626523-8-7.
- ^ Sternbach, L. H.; Reeder, E. (1961). "Quinazolines and 1,4-Benzodiazepines. IV.1,2 Transformations of 7-Chloro-2-methylamino-5-phenyl-3H-1,4-benzodiazepine 4-Oxide3". The Journal of Organic Chemistry 26 (12): 4936–4941. doi:10.1021/jo01070a038.
- ^ K.B. Nutley, L.H. Sternbach, U.S. Patent 3,109,843 (1963).
- ^ E. Reeder, L.H. Sternbach, U.S. Patent 3,371,085 (1968).
- ^ Gates, M. (1980). "New synthesis of diazepam". The Journal of Organic Chemistry 45 (9): 1675–1681. doi:10.1021/jo01297a030.
- ^ Ishikura, M. .; Mori, M. .; Ikeda, T. .; Terashima, M. .; Ban, Y. . (1982). "New synthesis of diazepam and the related 1,4-benzodiazepines by means of palladium-catalyzed carbonylation". The Journal of Organic Chemistry 47 (12): 2456–2461. doi:10.1021/jo00133a042.
- ^ a b Sample, Ian (October 3, 2005). "Leo Sternbach's Obituary". The Guardian (Guardian Unlimited). http://www.guardian.co.uk/medicine/story/0,,1583671,00.html. Retrieved 2006-03-10.
- ^ Marshall, KP.; Georgievskava, Z.; Georgievsky, I. (Jun 2009). "Social reactions to Valium and Prozac: a cultural lag perspective of drug diffusion and adoption". Res Social Adm Pharm 5 (2): 94–107. doi:10.1016/j.sapharm.2008.06.005. PMID 19524858.
- ^ Mant A; Whicker SD, McManus P, Birkett DJ, Edmonds D, Dumbrell D. (December 1993). "Benzodiazepine utilisation in Australia: report from a new pharmacoepidemiological database". Aust J Public Health. 17 (4): 345–9. doi:10.1111/j.1753-6405.1993.tb00167.x. PMID 7911332.
- ^ "New Evidence On Addiction To Medicines Diazepam Has Effect On Nerve Cells In The Brain Reward System". Medical News Today. August 2008. http://www.medicalnewstoday.com/articles/119284.php. Retrieved September 25, 2008.
- ^ Yoshimura K; Horiuchi M, Inoue Y, Yamamoto K. (January 1984). "[Pharmacological studies on drug dependence. (III): Intravenous self-administration of some CNS-affecting drugs and a new sleep-inducer, 1H-1, 2, 4-triazolyl benzophenone derivative (450191-S), in rats]". Nippon Yakurigaku Zasshi. 83 (1): 39–67. doi:10.1254/fpj.83.39. PMID 6538866.
- ^ Thiébot MH; Le Bihan C, Soubrié P, Simon P. (1985). "Benzodiazepines reduce the tolerance to reward delay in rats". Psychopharmacology (Berl). 86 (1–2): 147–52. doi:10.1007/BF00431700. PMID 2862657.
- ^ Woolverton WL, Nader MA (December 1995). "Effects of several benzodiazepines, alone and in combination with flumazenil, in rhesus monkeys trained to discriminate pentobarbital from saline". Psychopharmacology (Berl.) 122 (3): 230–6. doi:10.1007/BF02246544. PMID 8748392.
- ^ International Narcotics Control Board (1996). "CHAPTER II. OPERATION OF THE INTERNATIONAL DRUG CONTROL SYSTEM". REPORT OF THE INTERNATIONAL NARCOTICS CONTROL BOARD FOR 1996. http://www.incb.org/incb/en/annual_report_1996_chapter2.html#IIB10. Retrieved September 25, 2006.
- ^ Griffiths RR, Johnson MW (2005). "Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds". J Clin Psychiatry 66 Suppl 9: 31–41. PMID 16336040.
- ^ Overclocker. "Methamphetamine and Benzodiazepines: Methamphetamine & Benzodiazepines". Erowid Experience Vaults. http://de1.erowid.org/experiences/exp.phpquery=ID=9402.html. Retrieved September 26, 2006.
- ^ United States Government; U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES (2004). "Drug Abuse Warning Network, 2004: National Estimates of Drug-Related Emergency Department Visits". Substance Abuse and Mental Health Services Administration. Archived from the original on 31 March 2008. http://web.archive.org/web/20080331184508/http://dawninfo.samhsa.gov/files/DAWN2k4ED.htm. Retrieved 9 May 2008.
- ^ Bergman U; Dahl-Puustinen ML. (1989). "Use of prescription forgeries in a drug abuse surveillance network". Eur J Clin Pharmacol. 36 (6): 621–3. doi:10.1007/BF00637747. PMID 2776820.
- ^ Jones AW; Holmgren A, Kugelberg FC. (April 2007). "Concentrations of scheduled prescription drugs in blood of impaired drivers: considerations for interpreting the results". Ther Drug Monit. 29 (2): 248–60. doi:10.1097/FTD.0b013e31803d3c04. PMID 17417081.
- ^ Cosbey SH. (December 1986). "Drugs and the impaired driver in Northern Ireland: an analytical survey". Forensic Sci Int. 32 (4): 245–58. doi:10.1016/0379-0738(86)90201-X. PMID 3804143.
- ^ International Narcotics Control Board (2003). "List of psychotropic substances under international control" (PDF). Green list. http://www.incb.org/pdf/elist/green.pdf. Retrieved 2006-03-11.[dead link]
- ^ "Anlage III (zu § 1 Abs. 1) verkehrsfähige und verschreibungsfähige Betäubungsmittel". Betäubungsmittelgesetz. 2001. http://bundesrecht.juris.de/btmg_1981/anlage_iii_61.html. Retrieved 2010-01-05.
- ^ San Quentin State Prison Operational Procedure 0-770, Execution By Lethal Injection (pp. 43 & 92). http://www.cdcr.ca.gov/News/docs/RevisedProtocol.pdf
- ^ http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/190302.htm
- ^ Rahminiwati M, Nishimura M (April 1999). "Effects of delta 9-tetrahydrocannabinol and diazepam on feeding behavior in mice". The Journal of Veterinary Medical Science 61 (4): 351–5. doi:10.1292/jvms.61.351. PMID 10342284.
- ^ Hines, Ron DVM PhD (2006-01-14). "Epilepsy In Your Dog Or Cat". 2nd Chance Sanctuary Pet Health Center. http://www.2ndchance.info/epilepsy.htm. Retrieved May 18, 2006.
External links
- Roche Pharmaceuticals (AUS) - Valium Product Information
- U.S. National Library of Medicine: Drug Information Portal - Diazepam
- Flash animation about how bromazepam works (mechanism of action)
- Albany Medical Center – "Medication of the month"
Antidotes (V03AB) Nervous system Barbiturate overdoseBemegride • EthamivanBenzodiazepine overdoseGHB overdoseReversal of neuromuscular blockadeCardiovascular Other Paracetamol toxicity (Acetaminophen)OtherPrednisolone/promethazine • oxidizing agent (potassium permanganate) • iodine-131 (Potassium iodide) • Methylthioninium chloride#Emetic Ipecacuanha (Syrup of ipecac) • Copper sulfateAnticonvulsants (N03) GABAA receptor agonist Clobazam • Clonazepam • Clorazepate • Diazepam# • Flutoprazepam • Lorazepam • Midazolam • Nimetazepam • Nitrazepam • TemazepamOther GABA agents Carbonic anhydrase inhibitor Channel blockers Primarily sodiumPrimarily calciumUnknown/ungroupedChannel openers PotassiumRetigabineIndirect GABA agents GABA transaminase inhibitor: Valproic acid# (Sodium valproate & Valproate semisodium) • Valpromide • Valnoctamide • Valproate pivoxil
GABA reuptake inhibitor: TiagabineUnknown/multiple/
unsortedPropionatesAnxiolytics (N05B) GABAA PAMs Adinazolam • Alprazolam • Bretazenil • Bromazepam • Camazepam • Chlordiazepoxide • Clobazam • Clonazepam • Clorazepate • Clotiazepam • Cloxazolam • Diazepam • Ethyl Loflazepate • Etizolam • Fludiazepam • Halazepam • Imidazenil • Ketazolam • Lorazepam • Medazepam • Nordazepam • Oxazepam • Pinazepam • PrazepamAbecarnil • Adipiplon • Alpidem • CGS-8216 • CGS-9896 • CGS-13767 • CGS-20625 • Divaplon • ELB-139 • Fasiplon • GBLD-345 • Gedocarnil • L-838,417 • NS-2664 • NS-2710 • Ocinaplon • Pagoclone • Panadiplon • Pipequaline • RWJ-51204 • SB-205,384 • SL-651,498 • Taniplon • TP-003 • TP-13 • TPA-023 • Y-23684 • ZK-93423PyrazolopyridinesCartazolate • Etazolate • ICI-190,622 • TracazolateOthersChlormezanone • Ethanol (Alcohol) • Etifoxine • Kavalactones (Kava Kava) • Skullcap • Valerenic Acid (Valerian)α2δ VDCC Blockers 5-HT1A Agonists H1 Antagonists Diphenylmethanes: Captodiame • Hydroxyzine; Others: Brompheniramine • Chlorpheniramine • PheniramineCRH1 Antagonists NK2 Antagonists GR-159,897 • SaredutantMCH1 antagonists ATC-0175 • SNAP-94847mGluR2/3 Agonists mGluR5 NAMs TSPO agonists σ1 agonists Afobazole • OpipramolOthers Benzoctamine • Carbetocin • Demoxytocin • Mephenoxalone • Mepiprazole • Oxanamide • Oxytocin • Promoxolane • Tofisopam • Trimetozine • WAY-267,464Categories:- Benzodiazepines
- Hoffmann-La Roche
- World Health Organization essential medicines
- Lactams
- Organochlorides
- TSPO ligands
Wikimedia Foundation. 2010.