- Dimercaprol
-
Dimercaprol 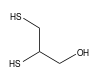
 2,3-Disulfanylpropan-1-ol[citation needed]Other names2,3-Dimercaptopropanol[citation needed]
2,3-Disulfanylpropan-1-ol[citation needed]Other names2,3-Dimercaptopropanol[citation needed]Identifiers CAS number 59-52-9  , 16495-08-2 (2R)-2-sulfanyl
, 16495-08-2 (2R)-2-sulfanyl  , 16495-16-2 (2S)-2-sulfanyl
, 16495-16-2 (2S)-2-sulfanyl 
PubChem 3080, 6971262 (2R)-2-sulfanyl, 3246063 (2S)-2-sulfanyl ChemSpider 2971  , 5342114 (2R)-2-sulfanyl
, 5342114 (2R)-2-sulfanyl  , 2496803 (2S)-2-sulfanyl
, 2496803 (2S)-2-sulfanyl 
UNII 0CPP32S55X 
EC number 200-433-7 UN number 2810 DrugBank DB06782 KEGG D00167 
MeSH Dimercaprol ChEMBL CHEMBL1597 
RTECS number UB2625000 ATC code V03 Beilstein Reference 1732058 Jmol-3D images Image 1 - OCC(S)CS
Properties Molecular formula C3H8S2O Molar mass 124.225 g mol-1 Exact mass 124.001656258 g mol-1 Density 1.239 g cm-3 Boiling point 120 °C, 393 K, 248 °F (at 2.0 kPa)
log P 0.627 Acidity (pKa) 8.999 Basicity (pKb) 4.998 Refractive index (nD) 1.573 Hazards GHS pictograms 
GHS signal word DANGER GHS hazard statements H301, H315, H319, H335 GHS precautionary statements P261, P301+310, P305+351+338 EU classification  Xn
XnR-phrases R22, R36/37/38 S-phrases S26, S36 NFPA 704 Flash point 112 °C Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Dimercaprol (INN) or British anti-Lewisite (abbreviated BAL), is a compound developed by British biochemists at Oxford University during World War II.[1][2] It was developed secretly as an antidote for lewisite, the now-obsolete arsenic-based chemical warfare agent.[3] Today, it is used medically in treatment of arsenic, mercury, gold and lead, and other toxic metal poisoning.[4] In addition, it has in the past been used for the treatment of Wilson's disease, a genetic disorder in which the body tends to retain copper.[5]
Biochemical function
Arsenic and some other heavy metals act by chemically reacting with adjacent thiol residues on metabolic enzymes, creating a chelate complex that inhibits the affected enzyme's activity.[6] Dimercaprol competes with the thiol groups for binding the metal ion, which is then excreted in the urine.[citation needed]
Dimercaprol is itself toxic, with a narrow therapeutic range and a tendency to concentrate arsenic in some organs. Other drawbacks include the need to administer it by painful intramuscular injection.[7] Serious side effects include nephrotoxicity and hypertension.
Dimercaprol has been found to form stable chelates in vivo with many other toxic metals including inorganic mercury, antimony, bismuth, cadmium, chromium, cobalt, gold, and nickel. However, it is not necessarily the treatment of choice for toxicity to these metals. Dimercaprol has been used as an adjunct in the treatment of the acute encephalopathy of lead toxicity. It is a potentially toxic drug, and its use may be accompanied by multiple side effects. Although treatment with dimercaprol will increase the excretion of cadmium, there is a concomitant increase in renal cadmium concentration, so that its use in case of cadmium toxicity is to be avoided. It does, however, remove inorganic mercury from the kidneys; but is not useful in the treatment of alkylmercury or phenyl mercury toxicity. Dimercaprol also enhances the toxicity of selenium and tellurium, so it is not to be used to remove these metals from the body.[citation needed]
See also
- Chelation therapy
- Chemical warfare agent
- Toxic metal poisoning
References
- ^ Domingo Tabangcura, Jr., G. Patrick Daubert. "British anti-Lewisite". http://www.chm.bris.ac.uk/motm/bal/development.html.
- ^ Peters, R; Stocken, L; Thompson, R. (1945). "British Anti-Lewisite (BAL)". Nature 156 (3969): 616–619. doi:10.1038/156616a0. PMID 21006485.
- ^ Domingo Tabangcura, Jr., G. Patrick Daubert. "British anti-Lewisite". http://www.chm.bris.ac.uk/motm/bal/development.html.
- ^ "Dimercaprol". http://www.drugs.com/dict/dimercaprol.html.
- ^ Denny-Brown D, PORTER H (December 1951). "The effect of BAL (2,3-dimercaptopropanol) on hepatolenticular degeneration (Wilson's disease)". N. Engl. J. Med. 245 (24): 917–25. doi:10.1056/NEJM195112132452401. PMID 14882450.
- ^ Goldman M, Dacre JC. (1989) Lewisite: its chemistry, toxicology, and biological effects. Rev Environ Contam Toxicol 110: 75-115
- ^ Mückter H, Liebl B, Reichl FX et al. (1997) Are we ready to replace dimercaprol (BAL) as an arsenic antidote? Human and Experimental Toxicology 16: 460-465
Antidotes (V03AB) Nervous system Barbiturate overdoseBemegride • EthamivanBenzodiazepine overdoseGHB overdoseReversal of neuromuscular blockadeCardiovascular Other Paracetamol toxicity (Acetaminophen)Dimercaprol# • SuccimerEdetates • Dimercaprol# • Prussian blue#OtherPrednisolone/promethazine • oxidizing agent (potassium permanganate) • iodine-131 (Potassium iodide) • Methylthioninium chloride#Emetic Ipecacuanha (Syrup of ipecac) • Copper sulfateChelating agents / chelation therapy (V03AC, others) Iron Copper Lead BAL# • EDTA# (Dexrazoxane)Thallium Other/ungrouped Categories:- Alcohols
- Antidotes
- Chelating agents
- Thiols
- World Health Organization essential medicines
Wikimedia Foundation. 2010.

