- Cobalt
-
This article is about the metal. For other uses, see Cobalt (disambiguation).
iron ← cobalt → nickel -
↑
Co
↓
RhAppearance hard lustrous gray metal

General properties Name, symbol, number cobalt, Co, 27 Pronunciation /ˈkoʊbɒlt/ koh-bolt[1] Element category transition metal Group, period, block 9, 4, d Standard atomic weight 58.933195(5) Electron configuration [Ar] 4s2 3d7 Electrons per shell 2, 8, 15, 2 (Image) Physical properties Color metallic gray Density (near r.t.) 8.90 g·cm−3 Liquid density at m.p. 7.75 g·cm−3 Melting point 1768 K, 1495 °C, 2723 °F Boiling point 3200 K, 2927 °C, 5301 °F Heat of fusion 16.06 kJ·mol−1 Heat of vaporization 377 kJ·mol−1 Molar heat capacity 24.81 J·mol−1·K−1 Vapor pressure P (Pa) 1 10 100 1 k 10 k 100 k at T (K) 1790 1960 2165 2423 2755 3198 Atomic properties Oxidation states 5, 4 , 3, 2, 1, -1[2]
(amphoteric oxide)Electronegativity 1.88 (Pauling scale) Ionization energies
(more)1st: 760.4 kJ·mol−1 2nd: 1648 kJ·mol−1 3rd: 3232 kJ·mol−1 Atomic radius 125 pm Covalent radius 126±3 (low spin), 150±7 (high spin) pm Miscellanea Crystal structure hexagonal Magnetic ordering ferromagnetic Electrical resistivity (20 °C) 62.4 nΩ·m Thermal conductivity 100 W·m−1·K−1 Thermal expansion (25 °C) 13.0 µm·m−1·K−1 Speed of sound (thin rod) (20 °C) 4720 m·s−1 Young's modulus 209 GPa Shear modulus 75 GPa Bulk modulus 180 GPa Poisson ratio 0.31 Mohs hardness 5.0 Vickers hardness 1043 MPa Brinell hardness 700 MPa CAS registry number 7440-48-4 Most stable isotopes Main article: Isotopes of cobalt iso NA half-life DM DE (MeV) DP 56Co syn 77.27 d ε 4.566 56Fe 57Co syn 271.79 d ε 0.836 57Fe 58Co syn 70.86 d ε 2.307 58Fe 59Co 100% 59Co is stable with 32 neutrons 60Co syn 5.2714 years β−, γ, γ 2.824 60Ni or /ˈkoʊbɔːlt/)[3] is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal. Cobalt-based blue pigments have been used since ancient times for jewelry and paints, and to impart a distinctive blue tint to glass, but the color was later thought by alchemists to be due to the known metal bismuth. Miners had long used the name kobold ore (German for goblin ore) for some of the blue-pigment producing minerals; they were named because they were poor in known metals and gave poisonous arsenic-containing fumes upon smelting. In 1735, such ores were found to be reducible to a new metal (the first discovered since ancient times), and this was ultimately named for the kobold.
Nowadays, some cobalt is produced specifically from various metallic-lustered ores, for example cobaltite (CoAsS), but the main source of the element is as a by-product of copper and nickel mining. The copper belt in the Democratic Republic of the Congo and Zambia yields most of the cobalt metal mined worldwide.
Cobalt is used in the preparation of magnetic, wear-resistant and high-strength alloys. Cobalt silicate and cobalt(II) aluminate (CoAl2O4, cobalt blue) give a distinctive deep blue color to glass, smalt, ceramics, inks, paints and varnishes. Cobalt occurs naturally as only one stable isotope, cobalt-59. Cobalt-60 is a commercially important radioisotope, used as a radioactive tracer and in the production of gamma rays.
Cobalt is the active center of coenzymes called cobalamin or vitamin B12, and is an essential trace element for all animals. Cobalt is also an active nutrient for bacteria, algae and fungi.
Contents
Characteristics
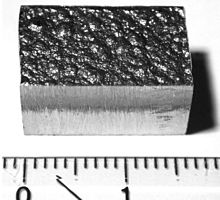 A block of electrolytically refined cobalt (99.9% purity) cut from a large plate
A block of electrolytically refined cobalt (99.9% purity) cut from a large plate
Cobalt is a ferromagnetic metal with a specific gravity of 8.9. Pure cobalt is not found in nature, but compounds of cobalt are common. Small amounts of it are found in most rocks, soil, plants and animals. The Curie temperature is 1115 °C[4] and the magnetic moment is 1.6–1.7 Bohr magnetons per atom.[5] In nature, it is frequently associated with nickel, and both are characteristic minor components of meteoric iron. Cobalt has a relative permeability two thirds that of iron.[6] Metallic cobalt occurs as two crystallographic structures: hcp and fcc. The ideal transition temperature between the hcp and fcc structures is 450 °C, but in practice, the energy difference is so small that random intergrowth of the two is common.[7][8][9]
Cobalt is a weakly reducing metal that is protected from oxidation by a passivating oxide film. It is attacked by halogens and sulfur. Heating in oxygen produces Co3O4 which loses oxygen at 900 °C to give the monoxide CoO.[10]
Compounds
See also Category: Cobalt compounds.Common oxidation states of cobalt include +2 and +3, although compounds with oxidation states ranging from −3 to +4 are also known. A common oxidation state for simple compounds is +2. Cobalt(II) salts form the red-pink [Co(H2O)6]2+ complex in aqueous solution. Addition of chloride gives the intensely blue [CoCl4]2−.[2]
Oxygen and chalcogen compounds
Several oxides of cobalt are known. Green cobalt(II) oxide (CoO) has rocksalt structure. It is readily oxidized with water and oxygen to brown cobalt(III) hydroxide (Co(OH)3). At temperatures of 600–700 °C, CoO oxidizes to the blue cobalt(II,III) oxide (Co3O4), which has a spinel structure.[2] Black cobalt(III) oxide (Co2O3) is also known.[11] Cobalt oxides are antiferromagnetic at low temperature: CoO (Neel temperature 291 K) and Co3O4 (Neel temperature: 40 K), which is analogous to magnetite (Fe3O4), with a mixture of +2 and +3 oxidation states.[12]
The principal chalcogenides of cobalt include the black cobalt(II) sulfides, CoS2, which adopts a pyrite-like structure, and Pentlandite (Co9S8) is metal-rich.[2]
Halides
The four dihalides of cobalt(II) are known: cobalt(II) fluoride (CoF2, pink), cobalt(II) chloride (CoCl2, blue), cobalt(II) bromide (CoBr2, green), cobalt(II) iodide (CoI2, blue-black). These halides exist as anhydrous and hydrates. Whereas the anhydrous dichloride is blue, the hydrate is red.[13]
The reduction potential for the reaction
- Co3+ + e− → Co2+
is +1.92 V, beyond that for chlorine to chloride, +1.36 V. As a consequence cobalt(III) and chloride would result in the cobalt(III) being reduced to cobalt(II). Because the reduction potential for fluorine to fluoride is so high, +2.87 V, cobalt(III) fluoride is one of the few simple stable cobalt(III) compounds. Cobalt(III) fluoride, which is used in some fluorination reactions, reacts vigorously with water.[10]
Coordination compounds
As for all metals, molecular compounds of cobalt are classified as coordination complexes, that is molecules or ions that contain cobalt linked to several ligands. The principles of electronegativity and hardness–softness of a series of ligands can be used to explain the usual oxidation state of the cobalt. For example Co+3 complexes tend to have ammine ligands. As phosphorus is softer than nitrogen, phosphine ligands tend to feature the softer Co2+ and Co+, an example being tris(triphenylphosphine)cobalt(I) chloride ((P(C6H5)3)3CoCl). The more electronegative (and harder) oxide and fluoride can stabilize Co4+ derivatives, e.g. caesium hexafluorocobaltate (Cs2CoF6) and potassium percobaltate (K3CoO4).[10]
Alfred Werner, a Nobel-prize winning pioneer in coordination chemistry, worked with compounds of empirical formula CoCl3(NH3)6. One of the isomers determined was cobalt(III) hexammine chloride. This coordination complex, a "typical" Werner-type complex, consists of a central cobalt atom coordinated by six ammine ligands orthogonal to each other and three chloride counteranions. Using chelating ethylenediamine ligands in place of ammonia gives tris(ethylenediamine)cobalt(III) chloride ([Co(en)3]Cl), which was one of the first coordination complexes that was resolved into optical isomers. The complex exists as both either right- or left-handed forms of a "three-bladed propeller". This complex was first isolated by Werner as yellow-gold needle-like crystals.[14][15]
Organometallic compounds
Main article: Organocobalt chemistryCobaltocene is a structural analog to ferrocene, where cobalt substitutes for iron. Cobaltocene is sensitive to oxidation, much more than ferrocene.[16] Cobalt carbonyl (Co2(CO)8) is a catalyst in carbonylation reactions.[17] Vitamin B12 (see below) is an organometallic compound found in nature and is the only vitamin to contain a metal atom.[18]
Isotopes
Main article: Isotopes of cobalt59Co is the only stable cobalt isotope and the only isotope to exist in nature. 22 radioisotopes have been characterized with the most stable being 60Co with a half-life of 5.2714 years, 57Co with a half-life of 271.79 days, 56Co with a half-life of 77.27 days, and 58Co with a half-life of 70.86 days. All of the remaining radioactive isotopes have half-lives that are shorter than 18 hours, and the majority of these are shorter than 1 second. This element also has 4 meta states, all of which have half-lives shorter than 15 minutes.[19]
The isotopes of cobalt range in atomic weight from 50 u (50Co) to 73 u (73Co). The primary decay mode for isotopes with atomic mass unit values less than that of the most abundant stable isotope, 59Co, is electron capture and the primary mode of decay for those of greater than 59 atomic mass units is beta decay. The primary decay products before 59Co are element 26 (iron) isotopes and the primary products after are element 28 (nickel) isotopes.[19]
History
Cobalt compounds have been used for centuries to impart a rich blue color to glass, glazes and ceramics. Cobalt has been detected in Egyptian sculpture and Persian jewelry from the third millennium BC, in the ruins of Pompeii (destroyed in 79 AD), and in China dating from the Tang dynasty (618–907 AD) and the Ming dynasty (1368–1644 AD).[20]
Cobalt has been used to color glass since the Bronze Age. The excavation of the Uluburun shipwreck yielded an ingot of blue glass, which was cast during the 14th century BC.[21][22] Blue glass items from Egypt are colored with copper, iron, or cobalt. The oldest cobalt-colored glass was from the time of the Eighteenth dynasty in Egypt (1550–1292 BC). The location where the cobalt compounds were obtained is unknown.[23][24]
The word cobalt is derived from the German kobalt, from kobold meaning "goblin", a superstitious term used for the ore of cobalt by miners. The first attempts at smelting these ores to produce metals such as copper or nickel failed, yielding simply powder (cobalt(II) oxide) instead. Also, because the primary ores of cobalt always contain arsenic, smelting the ore oxidized the arsenic content into the highly toxic and volatile arsenic oxide, which also decreased the reputation of the ore for the miners.[25]
Swedish chemist Georg Brandt (1694–1768) is credited with discovering cobalt circa 1735, showing it to be a new previously unknown element different from bismuth and other traditional metals, and calling it a new "semi-metal."[26][27] He was able to show that compounds of cobalt metal were the source of the blue color in glass, which previously had been attributed to the bismuth found with cobalt. Cobalt became the first metal to be discovered since the pre-historical period, during which all the known metals (iron, copper, silver, gold, zinc, mercury, tin, lead and bismuth) had no recorded discoverers.[28]
During the 19th century, a significant part of the world's production of cobalt blue (a dye made with cobalt compounds and alumina) and smalt (cobalt glass powdered for use for pigment purposes in ceramics and painting) was carried out at the Norwegian Blaafarveværket.[29][30] The first mines for the production of smalt in the 16th to 18th century were located in Norway, Sweden, Saxony and Hungary. With the discovery of cobalt ore in New Caledonia in 1864 the mining of cobalt in Europe declined. With the discovery of ore deposits in Ontario, Canada in 1904 and the discovery of even larger deposits in the Katanga Province in the Congo in 1914 the mining operations shifted again.[25] With the Shaba conflict starting in the 1978 the main source for cobalt the copper mines of Katanga Province nearly stopped their production.[31][32] The impact on the world cobalt economy from this conflict was smaller than expected, because industry established effective ways for recycling cobalt materials and in some cases was able to change to cobalt-free alternatives.[31][32]
In 1938, John Livingood and Glenn T. Seaborg discovered cobalt-60.[33] This isotope was famously used at Columbia University in the 1950s to establish parity violation in radioactive beta decay.[34][35]
Occurrence
The stable form of cobalt is created in supernovas via the r-process.[36] It comprises 0.0029% of the Earth's crust and is one of the first transition metal series.
Cobalt occurs in copper and nickel minerals and in combination with sulfur and arsenic in the sulfidic cobaltite (CoAsS), safflorite (CoAs2) and skutterudite (CoAs3) minerals.[10] The mineral cattierite is similar to pyrite and occurs together with vaesite in the copper deposits of the Katanga Province.[37] Upon contact with the atmosphere, weathering occurs and the sulfide minerals oxidize to form pink erythrite ("cobalt glance": Co3(AsO4)2·8H2O) and sphaerocobaltite (CoCO3).[38][39]
Cobalt is not found as a native metal but is mainly obtained as a by-product of nickel and copper mining activities. The main ores of cobalt are cobaltite, erythrite, glaucodot and skutterudite.[40][41]
Production
See also: Cobalt extraction techniquesIn 2005, the copper deposits in the Katanga Province (former Shaba province) of the Democratic Republic of the Congo were the top producer of cobalt with almost 40% world share, reports the British Geological Survey.[42] The political situation in the Congo influences the price of cobalt significantly.[43]
The Mukondo Mountain project, operated by the Central African Mining and Exploration Company in Katanga, may be the richest cobalt reserve in the world. It is estimated to be able to produce about one third of total global production of cobalt in 2008.[44] In July 2009 CAMEC announced a long term agreement under which CAMEC would deliver its entire annual production of cobalt in concentrate from Mukondo Mountain to Zhejiang Galico Cobalt & Nickel Materials of China.[45]
Several methods exist for the separation of cobalt from copper and nickel. They depend on the concentration of cobalt and the exact composition of the used ore. One separation step involves froth flotation, in which surfactants bind to different ore components, leading to an enrichment of cobalt ores. Subsequent roasting converts the ores to the cobalt sulfate, whereas the copper and the iron are oxidized to the oxide. The leaching with water extracts the sulfate together with the arsenates. The residues are further leached with sulfuric acid yielding a solution of copper sulfate. Cobalt can also be leached from the slag of the copper smelter.[46]
The products of the above-mentioned processes are transformed into the cobalt oxide (Co3O4). This oxide is reduced to the metal by the aluminothermic reaction or reduction with carbon in a blast furnace.[10]
Applications
The main application of cobalt is as the metal in alloys.[40][41]
Alloys
Cobalt-based superalloys consume most of the produced cobalt.[40][41] The temperature stability of these alloys makes them suitable for use in turbine blades for gas turbines and jet aircraft engines, though nickel-based single crystal alloys surpass them in this regard.[47] Cobalt-based alloys are also corrosion and wear-resistant. This makes them useful in the medical field, where cobalt is often used (along with titanium) for orthopedic implants that do not wear down over time. The development of the wear-resistant cobalt alloys started in the first decade of the 19th century with the stellite alloys, which are cobalt-chromium alloys with varying tungsten and carbon content. The formation of chromium and tungsten carbides makes them very hard and wear resistant.[48] Special cobalt-chromium-molybdenum alloys like Vitallium are used for prosthetic parts such as hip and knee replacements.[49] Cobalt alloys are also used for dental prosthetics, where they are useful to avoid allergies to nickel.[50] Some high speed steels also use cobalt to increase heat and wear-resistance. The special alloys of aluminium, nickel, cobalt and iron, known as Alnico, and of samarium and cobalt (samarium-cobalt magnet) are used in permanent magnets.[51] It is also alloyed with 95% platinum for jewelry purposes, yielding an alloy that is suitable for fine detailed casting and is also slightly magnetic.[52]
Batteries
Lithium cobalt oxide (LiCoO2) is widely used in lithium ion battery cathodes. The material is composed of cobalt oxide layers in which the lithium is intercalated. During discharging the lithium intercalated between the layers is set free as lithium ion.[53] Nickel-cadmium [54] (NiCd) and nickel metal hydride[55] (NiMH) batteries also contain significant amounts of cobalt; the cobalt improves the oxidation capabilities of nickel in the battery.[54]
Catalysis
Several cobalt compounds are used in chemical reactions as oxidation catalysts. Cobalt acetate is used for the conversion of xylene to terephthalic acid, the precursor to the bulk polymer polyethylene terephthalate. Typical catalysts are the cobalt carboxylates (known as cobalt soaps). They are also used in paints, varnishes, and inks as "drying agents" through the oxidation of drying oils.[53] The same carboxylates are used to improve the adhesion of the steel to rubber in steel-belted radial tires.
Cobalt-based catalysts are also important in reactions involving carbon monoxide. Steam reforming, useful in hydrogen production, uses cobalt oxide-base catalysts. Cobalt is also a catalyst in the Fischer-Tropsch process, used in the hydrogenation of carbon monoxide into liquid fuels.[56] The hydroformylation of alkenes often rely on cobalt octacarbonyl as the catalyst,[57] although such processes have been partially displaced by more efficient iridium- and rhodium-based catalysts, e.g. the Cativa process.
The hydrodesulfurization of petroleum uses a catalyst derived from cobalt and molybdenum. This process helps to rid petroleum of sulfur impurities that interfere with the refining of liquid fuels.[53]
Pigments and coloring
Before the 19th century, the predominant use of cobalt was as pigment. Since the Middle Ages, it has been involved in the production of smalt, a blue colored glass. Smalt is produced by melting a mixture of the roasted mineral smaltite, quartz and potassium carbonate, yielding a dark blue silicate glass which is ground after the production.[58] Smalt was widely used for the coloration of glass and as pigment for paintings.[59] In 1780, Sven Rinman discovered cobalt green and in 1802 Louis Jacques Thénard discovered cobalt blue.[60] The two varieties of cobalt blue, cobalt aluminate and cobalt green (a mixture of cobalt(II) oxide and zinc oxide), were used as pigments for paintings because of their superior stability.[61][62]
Radioisotopes
Cobalt-60 (Co-60 or 60Co) is useful as a gamma ray source because it can be produced in predictable quantity and high activity by bombarding cobalt with neutrons. It produces two gamma rays with energies of 1.17 and 1.33 MeV.[19][63]
Its uses include external beam radiotherapy, sterilization of medical supplies and medical waste, radiation treatment of foods for sterilization (cold pasteurization),[64] industrial radiography (e.g. weld integrity radiographs), density measurements (e.g. concrete density measurements), and tank fill height switches. The metal has the unfortunate habit of producing a fine dust, causing problems with radiation protection. Cobalt from radiotherapy machines has been a serious hazard when not disposed of properly, and one of the worst radiation contamination accidents in North America occurred in 1984, after a discarded radiotherapy unit containing cobalt-60 was mistakenly disassembled in a junkyard in Juarez, Mexico.[65][66]
Cobalt-60 has a radioactive half-life of 5.27 years. This decrease in activity requires periodic replacement of the sources used in radiotherapy and is one reason why cobalt machines have been largely replaced by linear accelerators in modern radiation therapy.[67]
Cobalt-57 (Co-57 or 57Co) is a cobalt radioisotope most often used in medical tests, as a radiolabel for vitamin B12 uptake, and for the Schilling test. Cobalt-57 is used as a source in Mössbauer spectroscopy and is one of several possible sources in X-ray fluorescence devices .[68][69]
Nuclear weapon designs could intentionally incorporate 59Co, some of which would be activated in a nuclear explosion to produce 60Co. The 60Co, dispersed as nuclear fallout, creates what is sometimes called a cobalt bomb.[70]
Other uses
Other uses of cobalt are in electroplating, owing to its attractive appearance, hardness and resistance to oxidation,[71] and as ground coats for porcelain enamels.[72]
Biological role
Cobalt is essential to all animals, including humans. It is a key constituent of cobalamin, also known as vitamin B12, which is the primary biological reservoir of cobalt as an "ultratrace" element. Bacteria in the guts of ruminant animals convert cobalt salts into vitamin B12, a compound which can only be produced by bacteria. The minimum presence of cobalt in soils therefore markedly improves the health of grazing animals, and an uptake of 0.20 mg/kg a day is recommended.[73] However, animals that do not have absorbable vitamin B12 produced by bacteria in their gastrointestinal tracts from cobalt salts in the diet must obtain the vitamin indirectly from animal products in their own diet, and cannot produce vitamin B12 by ingesting simple cobalt salts.
The cobalamin-based proteins use corrin to hold the cobalt. Coenzyme B12 features a reactive C-Co bond, which participates in its reactions.[74] In humans, B12 exists with two types of alkyl ligand: methyl and adenosyl. MeB12 promotes methyl (-CH3) group transfers. The adenosyl version of B12 catalyzes rearrangements in which a hydrogen atom is directly transferred between two adjacent atoms with concomitant exchange of the second substituent, X, which may be a carbon atom with substituents, an oxygen atom of an alcohol, or an amine. Methylmalonyl coenzyme A mutase (MUT) converts MMl-CoA to Su-CoA, an important step in the extraction of energy from proteins and fats.[75]
Although far less common than other metalloproteins (e.g. those of zinc and iron), cobaltoproteins are known aside from B12. These proteins include methionine aminopeptidase 2 an enzyme that occurs in humans and other mammals which does not use the corrin ring of B12, but binds cobalt directly. Another non-corrin cobalt enzyme is nitrile hydratase, an enzyme in bacteria that are able to metabolize nitriles.[76]
Precautions
Main article: Cobalt poisoningCobalt is an essential element for life in minute amounts. The LD50 value for soluble cobalt salts has been estimated to be between 150 and 500 mg/kg. Thus, for a 100 kg person the LD50 would be about 20 grams.[77]
After nickel and chromium, cobalt is a major cause of contact dermatitis.[78] In 1966, the addition of cobalt compounds to stabilize beer foam in Canada led to cardiomyopathy, which came to be known as beer drinker's cardiomyopathy.[79]
References
- ^ Oxford English Dictionary, 2nd Edition 1989.
- ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. pp. 1117–1119. ISBN 0080379419.
- ^ Wells, John C. (1990). Longman pronunciation dictionary. Harlow, England: Longman. p. 139. ISBN 0582053838. entry "cobalt"
- ^ Enghag, Per (2004). "Cobalt". Encyclopedia of the elements: technical data, history, processing, applications. p. 667. ISBN 9783527306664. http://books.google.com/books?id=aff7sEea39EC&pg=PA680.
- ^ Murthy, V. S. R (2003). "Magnetic Properties of Materials". Structure And Properties Of Engineering Materials. p. 381. ISBN 9780070482876. http://books.google.com/books?id=fi_rnPJeTV8C&pg=PA381.
- ^ Celozzi, Salvatore; Araneo, Rodolfo; Lovat, Giampiero (2008-05-01). Electromagnetic Shielding. pp. 27. ISBN 9780470055366. http://books.google.com/books?id=opQjaSj2yIMC&pg=PA27.
- ^ Lee, B.; Alsenz, R.; Ignatiev, A.; Van Hove, M. (1978). "Surface structures of the two allotropic phases of cobalt". Physical Review B 17 (4): 1510. Bibcode 1978PhRvB..17.1510L. doi:10.1103/PhysRevB.17.1510.
- ^ "Properties and Facts for Cobalt". http://www.americanelements.com/co.html. Retrieved 2008-09-19.
- ^ Cobalt, Centre d'Information du Cobalt, Brussels (1966). Cobalt. p. 45. http://books.google.com/books?id=H8XVAAAAMAAJ.
- ^ a b c d e Holleman, A. F., Wiberg, E., Wiberg, N. (2007). "Cobalt" (in German). Lehrbuch der Anorganischen Chemie, 102nd ed.. de Gruyter. pp. 1146–1152. ISBN 9783110177701.
- ^ Krebs, Robert E. (2006). The history and use of our earth's chemical elements: a reference guide (2 ed.). Greenwood Publishing Group. p. 107. ISBN 0313334382.
- ^ Petitto, Sarah C.; Marsh, Erin M.; Carson, Gregory A.; Langell, Marjorie A. (2008). "Cobalt oxide surface chemistry: The interaction of CoO(100), Co3O4(110) and Co3O4(111) with oxygen and water". Journal of Molecular Catalysis A: Chemical 281: 49. doi:10.1016/j.molcata.2007.08.023. http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1021&context=chemistrylangell.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. pp. 1119–1120. ISBN 0080379419.
- ^ Werner, A. (1912). "Zur Kenntnis des asymmetrischen Kobaltatoms. V". Chemische Berichte 45: 121–130. doi:10.1002/cber.19120450116.
- ^ Gispert, Joan Ribas (2008). "Early Theories of Coordination Chemistry". Coordination chemistry. pp. 31–33. ISBN 9783527318025. http://books.google.com/books?id=9d893122U6kC&pg=PR31.
- ^ James E. House (2008). Inorganic chemistry. Academic Press. pp. 767–. ISBN 9780123567864. http://books.google.com/books?id=ocKWuxOur-kC&pg=PA767. Retrieved 16 May 2011.
- ^ Charles M. Starks; Charles Leonard Liotta; Marc Halpern (1994). Phase-transfer catalysis: fundamentals, applications, and industrial perspectives. Springer. pp. 600–. ISBN 9780412040719. http://books.google.com/books?id=-QCGckdeKAkC&pg=PA600. Retrieved 16 May 2011.
- ^ Sigel, edited by Astrid (2010). Organometallics in Environment and Toxicology (Metal Ions in Life Sciences). Cambridge, UK: Royal Society of Chemistry Publishing. p. 75. ISBN 9781847551771.
- ^ a b c Audi, G. (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A (Atomic Mass Data Center) 729: 3–128. Bibcode 2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
- ^ Cobalt, Encyclopedia Britannica Online.
- ^ Pulak, Cemal (1998). "The Uluburun shipwreck: an overview". International Journal of Nautical Archaeology 27 (3): 188–224. doi:10.1111/j.1095-9270.1998.tb00803.x.
- ^ Henderson, Julian (2000). "Glass". The Science and Archaeology of Materials: An Investigation of Inorganic Materials. Routledge. p. 60. ISBN 9780415199339. http://books.google.com/?id=p9xJ-VpUuNkC.
- ^ Rehren, Th. (2003). "Aspects of the Production of Cobalt-blue Glass in Egypt". Archaeometry 43 (4): 483–489. doi:10.1111/1475-4754.00031.
- ^ Lucas, A. (2003). Ancient Egyptian Materials and Industries. Kessinger Publishing. p. 217. ISBN 9780766151413. http://books.google.com/?id=GugkliLHDMoC.
- ^ a b Dennis, W. H (2010). "Cobalt". Metallurgy: 1863–1963. pp. 254–256. ISBN 9780202363615. http://books.google.co/books?id=UyE49SzKWHIC&pg=PA254.
- ^ Georg Brandt first showed cobalt to be a new metal in: G. Brandt (1735) "Dissertatio de semimetallis" (Dissertation on semi-metals), Acta Literaria et Scientiarum Sveciae (Journal of Swedish literature and sciences), vol. 4, pages 1–10.
See also: (1) G. Brandt (1746) "Rön och anmärkningar angäende en synnerlig färg — cobolt" (Observations and remarks concerning an extraordinary pigment — cobalt), Kongliga Svenska vetenskapsakademiens handlingar (Transactions of the Royal Swedish Academy of Science), vol.7, pages 119–130; (2) G. Brandt (1748) “Cobalti nova species examinata et descripta” (Cobalt, a new element examined and described), Acta Regiae Societatis Scientiarum Upsaliensis (Journal of the Royal Scientific Society of Uppsala), 1st series, vol. 3 , pages 33–41; (3) James L. Marshall and Virginia R. Marshall (Spring 2003) "Rediscovery of the Elements: Riddarhyttan, Sweden," The Hexagon (official journal of the Alpha Chi Sigma fraternity of chemists), vol. 94, no. 1, pages 3–8. - ^ Wang, Shijie (2006). "Cobalt—Its recovery, recycling, and application". Journal of the Minerals, Metals and Materials Society 58 (10): 47–50. Bibcode 2006JOM....58j..47W. doi:10.1007/s11837-006-0201-y.
- ^ Weeks, Mary Elvira (1932). "The discovery of the elements. III. Some eighteenth-century metals". Journal of Chemical Education 9: 22. Bibcode 1932JChEd...9...22W. doi:10.1021/ed009p22.
- ^ Ivar B. Ramberg (2008). The making of a land: geology of Norway. Geological Society. pp. 98–. ISBN 9788292394427. http://books.google.com/books?id=rMVNE0F2SckC&pg=PA98. Retrieved 30 April 2011.
- ^ Cyclopaedia (1852). Cyclopædia of useful arts & manufactures, ed. by C. Tomlinson. 9 divs. pp. 400–. http://books.google.com/books?id=w_cGAAAAQAAJ&pg=PA400. Retrieved 30 April 2011.
- ^ a b Wellmer, Friedrich-Wilhelm; Becker-Platen, Jens Dieter. "Global Nonfuel Mineral Resources and Sustainability". United States Geological Survey. http://pubs.usgs.gov/circ/2007/1294/paper1.html.
- ^ a b Westing, Arthur H; Stockholm International Peace Research Institute (1986). "cobalt". Global resources and international conflict: environmental factors in strategic policy and action. pp. 75–78. ISBN 9780198291046. http://books.google.com/books?id=Xpypu9qqDncC&pg=PA75.
- ^ Livingood, J.; Seaborg, G. (1938). "Long-Lived Radio Cobalt Isotopes". Physical Review 53 (10): 847. Bibcode 1938PhRv...53..847L. doi:10.1103/PhysRev.53.847.
- ^ Wu, C. S. (1957). "Experimental Test of Parity Conservation in Beta Decay". Physical Review 105 (4): 1413. Bibcode 1957PhRv..105.1413W. doi:10.1103/PhysRev.105.1413.
- ^ Wróblewski, A.K. (2008). "The Downfall of Parity --- the Revolution That Happened Fifty Years Ago". Acta Physica Polonica B 39 (2): 251. Bibcode 2008AcPPB..39..251W. http://th-www.if.uj.edu.pl/acta/vol39/pdf/v39p0251.pdf.
- ^ Ptitsyn, D. A.; Chechetkin, V. M. (1980). "Creation of the Iron-Group Elements in a Supernova Explosion". Soviet Astronomy Letters 6: 61–64. Bibcode 1980SvAL....6...61P.
- ^ Kerr, Paul F. (1945). "Cattierite and Vaesite: New Co-Ni Minerals from the Belgian Kongo". American Mineralogist 30: 483–492. http://www.minsocam.org/ammin/AM30/AM30_483.pdf.
- ^ Buckley, A.N. (1987). "The Surface Oxidation of Cobaltite". Australian Journal of Chemistry 40 (2): 231. doi:10.1071/CH9870231.
- ^ Young, R (1957). "The geochemistry of cobalt". Geochimica et Cosmochimica Acta 13: 28. Bibcode 1957GeCoA..13...28Y. doi:10.1016/0016-7037(57)90056-X.
- ^ a b c Shedd, Kim B. "Mineral Yearbook 2006: Cobalt". United States Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/cobalt/myb1-2006-cobal.pdf. Retrieved 2008-10-26.
- ^ a b c Shedd, Kim B. "Commodity Report 2008: Cobalt". United States Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/cobalt/mcs-2008-cobal.pdf. Retrieved 2008-10-26.
- ^ "African Mineral Production". British Geological Survey. http://www.bgs.ac.uk/mineralsuk/downloads/african_mp_01_05.pdf. Retrieved 2009-06-06.
- ^ Wellmer, Friedrich-Wilhelm; Becker-Platen, Jens Dieter. "Global Nonfuel Mineral Resources and Sustainability". http://pubs.usgs.gov/circ/2007/1294/paper1.html. Retrieved 2009-05-16.
- ^ "CAMEC - The Cobalt Champion". International Mining. July 2008. http://www.infomine.com/publications/docs/InternationalMining/Chadwick2008t.pdf. Retrieved 2011-11-18.
- ^ Amy Witherden (6th July 2009). "Daily podcast - July 6, 2009". Mining weekly. http://www.miningweekly.com/article/daily-podcast---july-6-2009-2009-07-06-2. Retrieved 2011-11-15.
- ^ Davis, Joseph R. (2000). ASM specialty handbook: nickel, cobalt, and their alloys. ASM International. p. 347. ISBN 0871706857. http://books.google.com/?id=IePhmnbmRWkC&dq=cobalt+copper+nickel+ore+separate.
- ^ Donachie, Matthew J. (2002). Superalloys: A Technical Guide. ASM International. ISBN 9780871707499. http://books.google.com/?id=vjCJ5pI1QpkC.
- ^ Campbell, Flake C (2008-06-30). "Cobalt and Cobalt Alloys". Elements of metallurgy and engineering alloys. pp. 557–558. ISBN 9780871708670. http://books.google.com/books?id=6VdROgeQ5M8C&pg=PA557.
- ^ Michel, R.; Nolte, M.; Reich M.; Löer, F. (1991). "Systemic effects of implanted prostheses made of cobalt-chromium alloys". Archives of Orthopaedic and Trauma Surgery 110 (2): 61–74. doi:10.1007/BF00393876. PMID 2015136.
- ^ Disegi, John A. (1999). Cobalt-base Aloys for Biomedical Applications. ASTM International. p. 34. ISBN 0803126085. http://books.google.com/?id=z4rXM1EnPugC.
- ^ Luborsky, F. E.; Mendelsohn, L. I.; Paine, T. O. (1957). "Reproducing the Properties of Alnico Permanent Magnet Alloys with Elongated Single-Domain Cobalt-Iron Particles". Journal Applied Physics 28 (344): 344. Bibcode 1957JAP....28..344L. doi:10.1063/1.1722744.
- ^ . doi:10.1595/147106705X24409.
- ^ a b c Hawkins, M. (2001). "Why we need cobalt". Applied Earth Science: Transactions of the Institution of Mining & Metallurgy, Section B 110 (2): 66–71.
- ^ a b Armstrong, R. D.; Briggs, G. W. D.; Charles, E. A. (1988). "Some effects of the addition of cobalt to the nickel hydroxide electrode". Journal of Applied Electrochemistry 18 (2): 215. doi:10.1007/BF01009266.
- ^ Zhang, P (1999). "Recovery of metal values from spent nickel–metal hydride rechargeable batteries". Journal of Power Sources 77 (2): 116. doi:10.1016/S0378-7753(98)00182-7.
- ^ Khodakov, Andrei Y.; Chu, Wei and Fongarland, Pascal (2007). "Advances in the Development of Novel Cobalt Fischer-Tropsch Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels". Chemical Review 107 (5): 1692–1744. doi:10.1021/cr050972v.
- ^ Hebrard, Frédéric and Kalck, Philippe (2009). "Cobalt-Catalyzed Hydroformylation of Alkenes: Generation and Recycling of the Carbonyl Species, and Catalytic Cycle". Chemical Reviews 109 (9): 4272–4282. doi:10.1021/cr8002533. PMID 19572688.
- ^ Overman, Frederick (1852). A treatise on metallurgy. D. Appleton & company. pp. 631–637. http://books.google.com/?id=APgQAAAAIAAJ&pg=PA631.
- ^ Muhlethaler, Bruno; Thissen, Jean; Muhlethaler, Bruno (1969). "Smalt". Studies in Conservation 14 (2): 47–61. doi:10.2307/1505347. JSTOR 1505347.
- ^ Gehlen, A.F. (1803). "Ueber die Bereitung einer blauen Farbe aus Kobalt, die eben so schön ist wie Ultramarin. Vom Bürger Thenard". Neues allgemeines Journal der Chemie, Band 2 (H. Frölich.). http://books.google.com/?id=UGsMAQAAIAAJ&pg=RA1-PA506. (German translation from L. J. Thénard; Journal des Mines; Brumaire 12 1802; p 128-136
- ^ Witteveen, H. J.; Farnau, E. F. (1921). "Colors Developed by Cobalt Oxides". Industrial & Engineering Chemistry 13 (11): 1061. doi:10.1021/ie50143a048.
- ^ Venetskii, S. (1970). "The charge of the guns of peace". Metallurgist 14 (5): 334–336. doi:10.1007/BF00739447.
- ^ Mandeville, C.; Fulbright, H. (1943). "The Energies of the γ-Rays from Sb122, Cd115, Ir192, Mn54, Zn65, and Co60". Physical Review 64 (9–10): 265. Bibcode 1943PhRv...64..265M. doi:10.1103/PhysRev.64.265.
- ^ Wilkinson, V. M; Gould, G (1998). Food irradiation: a reference guide. p. 53. ISBN 9781855733596. http://books.google.com/books?id=FpIpsqs7CRUC&pg=PA53.
- ^ Blakeslee, Sandra (1984-05-01). "The Juarez accident". New York Times. http://query.nytimes.com/gst/fullpage.html?sec=health&res=9501E7D71338F932A35756C0A962948260. Retrieved 2009-06-06.
- ^ "Ciudad Juarez orphaned source dispersal, 1983". Wm. Robert Johnston. 2005-11-23. http://www.johnstonsarchive.net/nuclear/radevents/1983MEX1.html. Retrieved 2009-10-24.
- ^ National Research Council (U.S.). Committee on Radiation Source Use and Replacement; National Research Council (U.S.). Nuclear and Radiation Studies Board (January 2008). Radiation source use and replacement: abbreviated version. National Academies Press. pp. 35–. ISBN 9780309110143. http://books.google.com/books?id=3cT2REdXJ98C&pg=PA35. Retrieved 29 April 2011.
- ^ Meyer, Theresa (2001-11-30). Physical Therapist Examination Review. p. 368. ISBN 9781556425882. http://books.google.com/books?id=-gfKqUBGNgoC&pg=PA368.
- ^ Kalnicky, D; Singhvi, R (2001). "Field portable XRF analysis of environmental samples". Journal of Hazardous Materials 83 (1–2): 93–122. doi:10.1016/S0304-3894(00)00330-7. PMID 11267748.
- ^ Payne, L.R. (1977). "The Hazards of Cobalt". Occupational Medicine 27 (1): 20–25. doi:10.1093/occmed/27.1.20. http://occmed.oxfordjournals.org/cgi/content/abstract/27/1/20.
- ^ Davis, Joseph R; Handbook Committee, ASM International (2000-05-01). "Cobalt". Nickel, cobalt, and their alloys. p. 354. ISBN 9780871706850. http://books.google.com/books?id=IePhmnbmRWkC&pg=PA354.
- ^ Committee On Technological Alternatives For Cobalt Conservation, National Research Council (U.S.); National Materials Advisory Board, National Research Council (U.S.) (1983). "Ground–Coat Frit". Cobalt conservation through technological alternatives. p. 129. http://books.google.com/books?id=-CIrAAAAYAAJ&pg=PA129.
- ^ Schwarz, F. J.; Kirchgessner, M.; Stangl, G. I. (2000). "Cobalt requirement of beef cattle – feed intake and growth at different levels of cobalt supply". Journal of Animal Physiology and Animal Nutrition 83 (3): 121. doi:10.1046/j.1439-0396.2000.00258.x.
- ^ Voet, Judith G.; Voet, Donald (1995). Biochemistry. New York: J. Wiley & Sons. p. 675. ISBN 0-471-58651-X. OCLC 31819701.
- ^ Smith, David M.; Golding, Bernard T.; Radom, Leo (1999). "Understanding the Mechanism of B12-Dependent Methylmalonyl-CoA Mutase: Partial Proton Transfer in Action". Journal of the American Chemical Society 121 (40): 9388. doi:10.1021/ja991649a.
- ^ Kobayashi, Michihiko; Shimizu, Sakayu (1999). "Cobalt proteins". European Journal of Biochemistry 261 (1): 1–9. doi:10.1046/j.1432-1327.1999.00186.x. PMID 10103026.
- ^ Donaldson, John D. and Beyersmann, Detmar "Cobalt and Cobalt Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a07_281.pub2
- ^ Basketter, David A.; Angelini, Gianni; Ingber, Arieh; Kern, Petra S.; Menné, Torkil (2003). "Nickel, chromium and cobalt in consumer products: revisiting safe levels in the new millennium". Contact Dermatitis 49 (1): 1–7. doi:10.1111/j.0105-1873.2003.00149.x. PMID 14641113.
- ^ Barceloux, Donald G. and Barceloux, Donald (1999). "Cobalt". Clinical Toxicology 37 (2): 201–216. doi:10.1081/CLT-100102420.
External links
- National Pollutant Inventory (Australia)– Cobalt fact sheet
- London celebrates 50 years of Cobalt-60 Radiotherapy
- The periodic table of videos: Cobalt
Periodic table H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo Alkali metals Alkaline earth metals Lanthanides Actinides Transition metals Other metals Metalloids Other nonmetals Halogens Noble gases Unknown chem. properties Large version Cobalt compounds Categories:- Chemical elements
- Dietary minerals
- Transition metals
- Cobalt
- Ferromagnetic materials
- IARC Group 2B carcinogens
- Biology and pharmacology of chemical elements
Wikimedia Foundation. 2010.
Look at other dictionaries:
COBALT — Le cobalt (symbole Co, numéro atomique 27) est un métal placé sur la première ligne de la colonne VIII a, entre le fer et le nickel; il existe des analogies nombreuses entre cet élément, le rhodium et l’iridium. Le cobalt naturel, de masse… … Encyclopédie Universelle
cobalt — COBÁLT s.n. Element chimic metalic foarte dur, alb argintiu, întrebuinţat la fabricarea unor oţeluri speciale, în radioterapie etc., iar sărurile sale la colorarea în albastru a obiectelor de sticlă, de porţelan etc. ♦ Vas, obiect de sticlă, de… … Dicționar Român
Cobalt — Co balt (k[=o] b[o^]lt; 277, 74), n. [G. kobalt, prob. fr. kobold, kobel, goblin, MHG. kobolt; perh. akin to G. koben pigsty, hut, AS. cofa room, cofgodas household gods, Icel. kofi hut. If so, the ending old stands for older walt, wald, being… … The Collaborative International Dictionary of English
Cobalt — puede referirse a: Cobalt (Idaho) Cobalt (Misuri) Cobalt (Ontario) Chevrolet Cobalt, un modelo de automóvil. Cobalt Networks, una compañía de hardware de computadora. Palm OS Cobalt, versión 6.0 de Palm OS. Revista Cobalt, una revisa de novelas… … Wikipedia Español
cobalt — Symbol: Co Atomic number: 27 Atomic weight: 58.993 Light grey transition element. Some meteorites contain small amounts of metallic cobalt. Generally alloyed for use. Mammals require small amounts of cobalt salts. Cobalt 60, an artificially… … Elements of periodic system
cobalt 60 — sik stē n a heavy radioactive isotope of cobalt having the mass number 60 produced in nuclear reactors and used as a source of gamma rays esp. in place of radium (as in the treatment of cancer and in radiography) called also radiocobalt * * * a… … Medical dictionary
Cobalt — Cobalt, MO U.S. village in Missouri Population (2000): 189 Housing Units (2000): 89 Land area (2000): 0.145300 sq. miles (0.376326 sq. km) Water area (2000): 0.000000 sq. miles (0.000000 sq. km) Total area (2000): 0.145300 sq. miles (0.376326 sq … StarDict's U.S. Gazetteer Places
Cobalt, MO — U.S. village in Missouri Population (2000): 189 Housing Units (2000): 89 Land area (2000): 0.145300 sq. miles (0.376326 sq. km) Water area (2000): 0.000000 sq. miles (0.000000 sq. km) Total area (2000): 0.145300 sq. miles (0.376326 sq. km) FIPS… … StarDict's U.S. Gazetteer Places
cobalt-60 — noun A radioactive isotope of cobalt used as a source of gamma rays, eg in radiotherapy • • • Main Entry: ↑cobalt … Useful english dictionary
Cobalt 60 — is a Front 242 side project featuring Front 242 s Jean Luc de Meyer and Dominique Lallement. They are an electro industrial/EBM group, though they frequently use guitars, an uncommon feature among artists of the genre. Cobalt 60 has also done… … Wikipedia
cobalt — ► NOUN ▪ a hard silvery white metallic chemical element with magnetic properties, used in alloys. ORIGIN German Kobalt imp, demon (from the belief that cobalt was harmful to the ores with which it occurred) … English terms dictionary