- Group (periodic table)
-
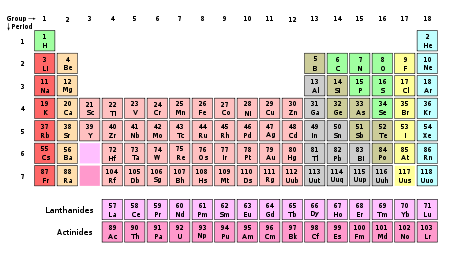
In chemistry, a group (also known as a family) is a vertical column in the periodic table of the chemical elements. There are 18 groups in the standard periodic table, including the d-block elements, but excluding the f-block elements.
The modern explanation of the pattern of the table is that the elements in a group have similar configurations of the outermost electron shells of their atoms (i.e. the same core charge), as most chemical properties are dominated by the orbital location of the outermost electron. There are three conventional ways of numbering: One using Arabic numerals, and two using Roman numerals. The Roman numeral names are the original traditional names of the groups; the Arabic numeral names are those recommended by the International Union of Pure and Applied Chemistry (IUPAC) to replace the old names in an attempt to reduce the confusion generated by the two older, but mutually confusing, schemes.
There is considerable confusion surrounding the two old systems in use (old IUPAC and CAS) that combined the use of Roman numerals with letters. Both systems agree on the Roman numerals, which indicate (approximately) the highest oxidation number of the elements in that group (and therefore indicates similar chemistry with other elements with the same Roman numeral), which proceeds in a linearly increasing fashion for the most part, once on the left of the table, and once on the right (see List of oxidation states of the elements), with some irregularities in the transition metals. However, the two systems use the letters differently.
In the old IUPAC system the letters A and B were designated to the left (A) and right (B) part of the table, while in the CAS system the letters A and B were designated to main group elements (A) and transition elements (B). The old IUPAC system was frequently used in Europe while the CAS was most common in America. The new IUPAC scheme was developed to replace both systems as they confusingly used the same names to mean different things. The new system simply numbers the groups increasingly from left to right on the standard periodic table. The IUPAC proposal was first circulated in 1985 for public comments,[1] and was later included as part of the 1990 edition of the Nomenclature of Inorganic Chemistry.[2]
Contents
Groups
The periodic table groups are as follows:
New IUPAC numbering Old IUPAC (European) CAS (American) Name Group 1 IA IA the alkali metals or lithium family Group 2 IIA IIA the alkaline earth metals or beryllium family Group 3 IIIA IIIB the scandium family (consisting of the rare earth elements plus the actinides) Group 4 IVA IVB the titanium family Group 5 VA VB the vanadium family Group 6 VIA VIB the chromium family Group 7 VIIA VIIB the manganese family Group 8 VIII VIIIB the iron family Group 9 VIII VIIIB the cobalt family Group 10 VIII VIIIB the nickel family Group 11 IB IB the coinage metals (not an IUPAC-recommended name) or copper family Group 12 IIB IIB the zinc family Group 13 IIIB IIIA the boron group or boron family Group 14 IVB IVA the carbon group or carbon family Group 15 VB VA the pnictogens or nitrogen family Group 16 VIB VIA the chalcogens or oxygen family Group 17 VIIB VIIA the halogens or fluorine family Group 18 Group 0 VIIIA the noble gases or helium family or neon family References
Footnotes
- ^ Fluck, E. New notations in the periodic table. Pure & App. Chem. 1988, 60, 431-436.[1]
- ^ Leigh, G. J. Nomenclature of Inorganic Chemistry: Recommendations 1990. Blackwell Science, 1990. ISBN 0632024941.
Background
- Scerri, E. R. The Periodic Table, Its Story and Its Significance, Oxford University Press, 2007. ISBN 978-0-19-530573-9.
Periodic tables Layouts - Standard
- Large table
- Inline f-block
- Vertical
- Text only
- Metals and nonmetals
- Blocks
- Valences
- Extension beyond the 7th period
- Large extended table
- Electron configurations
- Electronegativities
- Alternatives
- Crystal structure
- Discovery periods
List of elements by - Name etymology (symbol)
- Discovery
- Oxidation state
- Abundance (in humans)
- Nuclear stability
- Hardness
- Atomic properties
- Production
Groups Other element categories - Periods
- Metals
- Transition metals
- Metalloids
- Nonmetals
- Lanthanides
- Actinides
- Rare earth elements
- Platinum group metals (PGMs)
- Post-transition metals
- Refractory metals
Blocks Periods Categories:- Chemical element groups
- Periodic table
Wikimedia Foundation. 2010.