- Cobaltocene
-
Cobaltocene 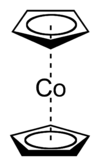
 cobaltocene,
cobaltocene,
bis(η5- cyclopentadienyl)-
cobaltOther namesCp2CoIdentifiers CAS number 1277-43-6 
ChemSpider 83848 
ChEBI CHEBI:30678 
RTECS number GG0350000 Jmol-3D images Image 1 - [cH-]1cccc1.[cH-]1cccc1.[Co+2]
Properties Molecular formula [Co(η5C5H5)2] Molar mass 189.12 g/mol Solubility in water not soluble Structure Coordination
geometrysandwich Dipole moment zero Thermochemistry Std enthalpy of
formation ΔfHo298+237 kJ/mol (uncertain) Std enthalpy of
combustion ΔcHo298-5839 kJ/mol Standard molar
entropy So298236 J.K−1.mol−1 Hazards EU classification  Xi
XiR-phrases R10, R36/37/38 S-phrases S9, S16, S26, S33, S37/39 NFPA 704 Related compounds Related metallocenes Ferrocene
Nickelocene
Rhodocene (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cobaltocene, known also as bis(cyclopentadienyl)cobalt(II) or even "bis Cp cobalt", is an organocobalt compound with the formula Co(C5H5)2. It is a dark purple solid that sublimes readily slightly above room temperature. Cobaltocene was discovered shortly after ferrocene, the first metallocene. Due to the ease with which it reacts with O2, the compound must be handled and stored using air-free techniques.
Contents
Synthesis
Cobaltocene is prepared by the reaction of sodium cyclopentadienide (NaC5H5) with anhydrous cobalt(II) chloride in THF solution. Sodium chloride is cogenerated, and the organometallic product is usually purified by vacuum sublimation.[1]
Structure and bonding
In Co(C5H5)2 the Co centre is "sandwiched" between two cyclopentadienyl (Cp) rings. The Co-C bond lengths are about 2.1 Å, slightly longer than the Fe-C bond in ferrocene.[2]
Co(C5H5)2 belongs to a group of organometallic compounds called metallocenes or sandwich compounds.[3]Cobaltocene has 19 valence electrons, one more than usually found in organotransition metal complexes, such as its very stable relative ferrocene. (See 18-electron rule.) This additional electron occupies an orbital that is antibonding with respect to the Co-C bonds. Consequently, the Co-C distances are slightly longer than the Fe-C bonds in ferrocene. Many chemical reactions of Co(C5H5)2 are characterized by its tendency lose this "extra" electron, yielding 18-electron cation known as cobaltocenium:
-
2Co(C5H5)2 + I2 → 2[Co(C5H5)2]+ + 2I− 19e− 18e−
The otherwise close relative of cobaltocene, rhodocene does not exist as a monomer, but spontaneously dimerizes by formation of a C-C bond between Cp rings.
Reactions
Redox properties
Co(C5H5)2 is a common one-electron reducing agent in the laboratory.[4] In fact, the reversibility of the Co(C5H5)2 redox couple is so well behaved that Co(C5H5)2 may be used in cyclic voltammetry as an internal standard. Its permethylated analogue decamethylcobaltocene (Co(C5Me5)2) is an especially powerful reducing agent, due to inductive donation of electron density from the 10 methyl groups, prompting the cobalt to give up its "extra" electron even more so. These two compounds are rare examples of reductants that dissolve in non-polar organic solvents. The reduction potentials of these compounds follow, using the ferrocene-ferrocenium couple as the reference:
- Fe(C5H5)2+/Fe(C5H5)2: 0 V
- Fe(C5Me5)2+/Fe(C5Me5)2: -0.59
- Co(C5H5)2+/Co(C5H5)2: -1.33
- Co(C5Me5)2+/Co(C5Me5)2: -1.94
The data show that the decamethyl compounds are ca. 600 mV more reducing than the parent metallocenes. This substituent effect is, however, overshadowed by the influence of the metal: changing from Fe to Co renders the reduction more favorable by over 1.3 volts.
Carbonylation
Treatment of Co(C5H5)2 with carbon monoxide gives the cobalt(I) derivative Co(C5H5)(CO)2, concomitant with loss of one Cp ligand.[1]
References
- ^ a b King, R. B. “Organometallic Syntheses” Volume 1: Academic Press: New York, 1965.
- ^ M. Yu. Antipin, R. Boese, N. Augart, G. Schmid "Redetermination of the cobaltocene crystal structure at 100 K and 297 K: Comparison with ferrocene and nickelocene" Structural Chemistry 1993, Volume 4, Number 2, 91-101. doi:10.1007/BF00677370
- ^ C. Elschenbroich, A. Salzer ”Organometallics : A Concise Introduction” (2nd Ed) (1992) from Wiley-VCH: Weinheim. ISBN 3-527-28165-7
- ^ N. G. Connelly, W. E. Geiger (1996). "Chemical Redox Agents for Organometallic Chemistry". Chem. Rev. 96 (2): 877–910. doi:10.1021/cr940053x. PMID 11848774.
External links
Categories:- Organocobalt compounds
- Metallocenes
- IARC Group 2B carcinogens
Wikimedia Foundation. 2010.

