- Sodium cyclopentadienide
-
Sodium cyclopentadienide 
 Other namessodium cyclopentadienylide, cyclopentadienylsodium
Other namessodium cyclopentadienylide, cyclopentadienylsodiumIdentifiers CAS number 4984-82-1 
PubChem 78681 ChemSpider 71032 
EC number 225-636-8 Jmol-3D images Image 1 - [Na+].c1[c-]ccc1
Properties Molecular formula C5H5Na Molar mass 88.08 g mol−1 Solubility in water Reacts Solubility in tetrahydrofuran Soluble  cyclopentadienide (verify) (what is:
cyclopentadienide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp or CpNa, where Cp− is the cyclopentadienide anion. Cp is also used as an abbreviation for the cyclopentadienyl ligand in coordination chemistry.[1]
Preparation
Sodium cyclopentadienide is commercially available as a solution in THF. It is prepared by treating cyclopentadiene with sodium:[2]
- 2 Na + 2 C5H6 → 2 NaC5H5 + H2
Commonly, the conversion is conducted by heating a suspension of molten sodium in dicyclopentadiene.[3] In former times, the sodium was provided in the form of "sodium wire" or "sodium sand", a fine dispersion of sodium prepared by melting sodium in refluxing xylene and rapidly stirring, was common.[4][5] Sodium hydride is a convenient base:[6]
- NaH + C5H6 → NaC5H5 + H2
In early work, Grignard reagents were used as bases. With a pKa of 15, cyclopentadiene can be deprotonated by many reagents.
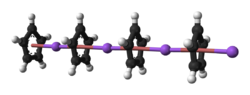
The nature of NaCp depends strongly its medium and for the purposes of planning syntheses, the reagent is often represented as a salt Na+C5H5−. Crystalline solvent-free NaCp, which is rarely encountered, is a "polydecker" sandwich complex, consisting of an infinite chain of alternating Na+ centers sandwiched between μ-η5:η5-C5H5 ligands.[7] As a solution in donor solvents, NaCp is highly solvated, especially at the alkali metal as suggested by the isolability of the adduct Na(tmeda)Cp.[8]
Applications
Sodium cyclopentadienide is a common reagent for the preparation of metallocenes. For example, the preparation of ferrocene[4] and zirconocene dichloride:[9]
- 2 NaC5H5 + FeCl2 → Fe(C5H5)2 + 2 NaCl
- ZrCl4(thf)2 + 2 NaCp → Cp2ZrCl2 + 2 NaCl + 2 THF
References
- ^ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 262. Electronic version.
- ^ Cotton, F. Albert; Wilkinson, Geoffrey (1988), Advanced Inorganic Chemistry (5th ed.), New York: Wiley-Interscience, p. 139, ISBN 0-471-84997-9
- ^ Tarun K. Panda, Michael T. Gamer, Peter W. Roesky "An Improved Synthesis of Sodium and Potassium Cyclopentadienide" Organometallics, 2003, 22, 877–878.doi:10.1021/om0207865
- ^ a b Wilkinson, Geoffrey (1963), "Ferrocene", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4p0473; Coll. Vol. 4: 473
- ^ Partridge, John J.; Chadha, Naresh K.; Uskokovic, Milan R. (1990), "An asymmetric hydroboration of 5-substituted cyclopentadienes: synthesis of methyl (1R,5R)-5-hydroxy-2-cyclopentene-1-acetate", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv7p0339; Coll. Vol. 7: 339
- ^ Girolami, G. S.; Rauchfuss, T. B. and Angelici, R. J., Synthesis and Technique in Inorganic Chemistry, University Science Books: Mill Valley, CA, 1999.ISBN 0935702482
- ^ Robert E. Dinnebier, Ulrich Behrens, and Falk Olbrich (1997). "Solid State Structures of Cyclopentadienyllithium, -sodium, and -potassium. Determination by High-Resolution Powder Diffraction". Organometallics 16: 3855–3858. doi:10.1021/om9700122.
- ^ Elschenbroich, C. ”Organometallics” (2006) Wiley-VCH: Weinheim. ISBN 978-3-29390-6
- ^ Wilkinson, G.; Birmingham, J. G. (1954). "Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta". J. Am. Chem. Soc. 76 (17): 4281–84. doi:10.1021/ja01646a008.
Categories:- Organosodium compounds
- Cyclopentadienyl complexes
Wikimedia Foundation. 2010.
