- Cyclopentadienylcobalt dicarbonyl
-
Cyclopentadienyl cobalt dicarbonyl 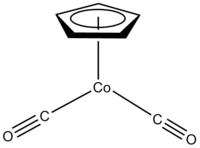
Identifiers CAS number 12078025-0 
Properties Molecular formula CpCo(CO)2 Molar mass 180.05 g/mol Appearance Dark red to black liquid Density 1.35 g/cm3 Melting point -22 °C, 251 K, -8 °F
Boiling point 139-140 °C (710 mmHg)
37-38.5 °C (2 mmHg)Solubility in water Insoluble Hazards R-phrases R10 R23/24/25 R42/43 R53 S-phrases S23 S36/37 S45  dicarbonyl (verify) (what is:
dicarbonyl (verify) (what is:  /
/ ?)
?)
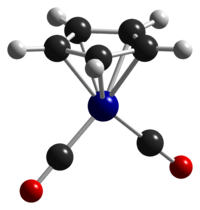
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cyclopentadienylcobalt dicarbonyl is an organocobalt compound with formula (C5H5)Co(CO)2, abbreviated CpCo(CO)2. It is an example of a half-sandwich complex. It is a dark red air sensitive liquid. This compound features one cyclopentadienyl ring that is bound in an η5-manner and two carbonyl ligands. The compound is soluble in common organic solvents and it is best stored under nitrogen.[1]
Preparation
CpCo(CO)2 was first reported in 1954 by Piper, Cotton, and Wilkinson who produced it by the reaction of cobalt carbonyl with cyclopentadiene.[2] It is prepared commercially by the same method:
- Co2(CO)8 + 2 C5H6 → 2 C5H5Co(CO)2 + H2 + 4 CO
Alternatively, it is generated by the high pressure carbonylation of bis(cyclopentadienyl)cobalt (cobaltocene) at elevated temperature and pressures:
- Co(C5H5)2 + 2 CO → C5H5Co(CO)2 + "C5H5"
Reactions
CpCo(CO)2 catalyzes the cyclotrimerization of alkynes.[3] The catalytic cycle begins with dissociation of one Co ligand forming bis(alkyne) intermediate.[4]
- CpCo(CO)2 + 2 R2C2 → CpCo(R2C2)2 + 2 CO
This reaction proceeds by coordination of one alkyne, followed by dissociation of the second CO. Although monoalkyne complexes CpCo(CO)(R1C2R2) have not been isolated, their analogs, CpCo(PPh3)(R1C2R2) are known. They are accessible, inter alia, by the following reactions:[4]
- CpCo(CO)2 + PR3 → CO + CpCo(CO)(PR3)
- CpCoL(PR3) + R2C2 → L + CpCo(PR3)(R2C2) (where L = CO or PR3)
CpCo(CO)2 catalyzes the formation of pyridines from a mixture of alkynes and nitriles. Reduction of CpCo(CO)2 with sodium yields the dinuclear radical [Cp2Co2(CO)2]-, which reacts with alkyl halides to give the dialkyl complexes [Cp2Co2(CO)2R2]. Ketones are produced by carbonylation of these dialkyl complexes, regenerating CpCo(CO)2.[4]
References
- ^ King, R.B.; Stone, F.G.A (1963). "Cyclopentadienyl Metal Carbonyls and Some Derivatives". Inorg. Synth. 7: 99. doi:10.1002/9780470132388.ch31.
- ^ Piper, T.S.; Cotton, F.A.; Wilkinson, G. (1955). "Cyclopentadienyl-carbon monoxide and related compounds of some transitional metals.". Journal of Inorganic and Nuclear Chemistry 1 (3): 165. doi:10.1016/0022-1902(55)80053-X.
- ^ Staeb, T.H.; Chavez, J.; Gleiter, R.; Nuber, Bernhard (2005). "The Role of [η2-Bis(tert-butylsulfonyl)acetylene](carbonyl)(η5-cyclopentadienyl) cobalt(I) as an Intermediate in the Alkyne Dimerisation". Eur. J. Inorg. Chem. 2005 (20): 4090. doi:10.1002/ejic.200500394.
- ^ a b c Pauson, P.L. “Dicarbonyl(cyclopentadienyl)cobalt(I).” Encyclopedia of Reagents for Organic Synthesis. 2001. doi:10.1002/047084289X.rd078
Categories:- Cyclopentadienyl complexes
- Carbonyl complexes
- Organocobalt compounds
Wikimedia Foundation. 2010.
