- Atomic radius
-
The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius.
Depending on the definition, the term may apply only to isolated atoms, or also to atoms in condensed matter, covalently bound in molecules, or in ionized and excited states; and its value may be obtained through experimental measurements, or computed from theoretical models. Under some definitions, the value of the radius may depend on the atom's state and context.[1]
The concept is difficult to define because the electrons do not have definite orbits, or sharply defined ranges. Rather, their positions must be described as probability distributions that taper off gradually as one moves away from the nucleus, without a sharp cutoff. Moreover, in condensed matter and molecules, the electron clouds of the atoms usually overlap to some extent, and some of the electrons may roam over a large region encompassing two or more atoms.
Despite these conceptual difficulties, under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 angstroms. Therefore, the radius of an atom is more than 10,000 times the radius of its nucleus (1–10 fm),[2] and less than 1/1000 of the wavelength of visible light (400–700 nm).
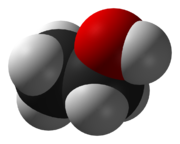 The approximate shape of a molecule of ethanol, CH3CH2OH. Each atom is modeled by a sphere with the element's Van der Waals radius.
The approximate shape of a molecule of ethanol, CH3CH2OH. Each atom is modeled by a sphere with the element's Van der Waals radius.
For many purposes, atoms can be modeled as spheres. This is only a crude approximation, but it can provide quantitative explanations and predictions for many phenomena, such as the density of liquids and solids, the diffusion of fluids through molecular sieves, the arrangement of atoms and ions in crystals, and the size and shape of molecules.[citation needed]
Atomic radii vary in a predictable and explicable manner across the periodic table. For instance, the radii generally decrease along each period (row) of the table, from the alkali metals to the noble gases; and increase down each group (column). The radius increases sharply between the noble gas at the end of each period and the alkali metal at the beginning of the next period. These trends of the atomic radii (and of various other chemical and physical properties of the elements) can be explained by the electron shell theory of the atom; they provided important evidence for the development and confirmation of quantum theory.
Contents
History
In 1920, shortly after it had become possible to determine the sizes of atoms using X-ray crystallography, it was suggested that all atoms of the same element have the same radii.[3] However, in 1923, when more crystal data had become available, it was found that an atom does not have just one size. For example, the size of lithium atoms is different in lithium metal than in LiCl.[4]
Definitions
Widely used definitions of atomic radius include:
- Van der Waals radius: in principle, half the minimum distance between the nuclei of two atoms of the element that are not bound to the same molecule.[5]
- Ionic radius: the nominal radius of the ions of an element in a specific ionization state, deduced from the spacing of atomic nuclei in crystalline salts that include that ion. In principle, the spacing between two adjacent oppositely charged ions (the length of the ionic bond between them) should equal the sum of their ionic radii.[5]
- Covalent radius: the nominal radius of the atoms of an element when covalently bound to other atoms, as deduced the separation between the atomic nuclei in molecules. In principle, the distance between two atoms that are bound to each other in a molecule (the length of that covalent bond) should equal the sum of their covalent radii.[5]
- Metallic radius: the nominal radius of atoms of an element when joined to other atoms by metallic bonds.[citation needed]
- Bohr radius: the radius of the lowest-energy electron orbit predicted by Bohr model of the atom (1913).[6][7] It is only applicable to atoms and ions with a single electron, such as hydrogen, singly ionized helium, and positronium. Although the model itself is now obsolete, the Bohr radius for the hydrogen atom is still regarded as an important physical constant.
Empirically measured atomic radii
The following table shows empirically measured covalent radii for the elements, as published by J. C. Slater in 1964.[8] The values are in picometers (pm), with an accuracy of about 5 pm. The shade of the box ranges from red to yellow as the radius increases; gray indicates lack of data.
Group (vertical) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Period (horizontal) 1 H
25He
2 Li
145Be
105B
85C
70N
65O
60F
50Ne
3 Na
180Mg
150Al
125Si
110P
100S
100Cl
100Ar
4 K
220Ca
180Sc
160Ti
140V
135Cr
140Mn
140Fe
140Co
135Ni
135Cu
135Zn
135Ga
130Ge
125As
115Se
115Br
115Kr
5 Rb
235Sr
200Y
180Zr
155Nb
145Mo
145Tc
135Ru
130Rh
135Pd
140Ag
160Cd
155In
155Sn
145Sb
145Te
140I
140Xe
6 Cs
260Ba
215*
Hf
155Ta
145W
135Re
135Os
130Ir
135Pt
135Au
135Hg
150Tl
190Pb
180Bi
160Po
190At
Rn
7 Fr
Ra
215**
Rf
Db
Sg
Bh
Hs
Mt
Ds
Rg
Cn
Uut
Uuq
Uup
Uuh
Uus
Uuo
Lanthanides *
La
195Ce
185Pr
185Nd
185Pm
185Sm
185Eu
185Gd
180Tb
175Dy
175Ho
175Er
175Tm
175Yb
175Lu
175Actinides **
Ac
195Th
180Pa
180U
175Np
175Pu
175Am
175Cm
Bk
Cf
Es
Fm
Md
No
Lr
Explanation of the general trends
The way the atomic radius varies with increasing atomic number can be explained by the arrangement of electrons in shells of fixed capacity. The shells are generally filled in order of increasing radius, since the negatively charged electrons are attracted by the positively charged protons in the nucleus. As the atomic number increases along each row of the periodic table, the additional electrons go into the same outermost shell; whose radius gradually contracts, due to the increasing nuclear charge. In a noble gas, the outermost shell is completely filled; therefore, the additional electron of next alkali metal will go into the next outer shell, accounting for the sudden increase in the atomic radius.
The increasing nuclear charge is partly counterbalanced by the increasing number of electrons, a phenomenon that is known as shielding; which explains why the size of atoms usually increases down each column. However, there are two occasions where shielding is less effective: in these cases, the atoms are smaller than would otherwise be expected.[clarification needed]
The following table summarizes the main phenomena that influence the atomic radius of an element:
factor principle increase with... tend to effect on radius electron shells quantum mechanics principal and azimuthal quantum numbers increase atomic radius increases down each column nuclear charge attractive force acting on electrons by protons in nucleus atomic number decrease atomic radius decreases along each period shielding repulsive force acting on outermost shell electrons by inner electrons number of electron shells increase atomic radius reduces the effect of the 2nd factor Lanthanide contraction
Main article: Lanthanide contractionThe electrons in the 4f-subshell, which is progressively filled from cerium (Z = 58) to lutetium (Z = 71), are not particularly effective at shielding the increasing nuclear charge from the sub-shells further out. The elements immediately following the lanthanides have atomic radii which are smaller than would be expected and which are almost identical to the atomic radii of the elements immediately above them.[9] Hence hafnium has virtually the same atomic radius (and chemistry) as zirconium, and tantalum has an atomic radius similar to niobium, and so forth. The effect of the lanthanide contraction is noticeable up to platinum (Z = 78), after which it is masked by a relativistic effect known as the inert pair effect.
Due to lanthanide contraction, the 5 following observations can be drawn:
- The size of Ln3+ ions regularly decreases with atomic number. According to Fajans' rules, decrease in size of Ln3+ ions increases the covalent character and decreases the basic character between Ln3+ and OH− ions in Ln(OH)3. Hence the order of size of Ln3+ is given:
La3+ > Ce3+ > ... , ... > Lu3+. - There is a regular decrease in their ionic radii.
- There is a regular decrease in their tendency to act as a reducing agent, with increase in atomic number.
- The second and third rows of d-block transition elements are quite close in properties.
- Consequently, these elements occur together in natural minerals and are difficult to separate.
d-Block contraction
Main article: d-block contractionThe d-block contraction is less pronounced than the lanthanide contraction but arises from a similar cause. In this case, it is the poor shielding capacity of the 3d-electrons which affects the atomic radii and chemistries of the elements immediately following the first row of the transition metals, from gallium (Z = 31) to bromine (Z = 35).[9]
Calculated atomic radii
The following table shows atomic radii computed from theoretical models, as published by Enrico Clementi and others in 1967.[10] The values are in picometres (pm).
Group (vertical) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Period (horizontal) 1 H
53He
312 Li
167Be
112B
87C
67N
56O
48F
42Ne
383 Na
190Mg
145Al
118Si
111P
98S
88Cl
79Ar
714 K
243Ca
194Sc
184Ti
176V
171Cr
166Mn
161Fe
156Co
152Ni
149Cu
145Zn
142Ga
136Ge
125As
114Se
103Br
94Kr
885 Rb
265Sr
219Y
212Zr
206Nb
198Mo
190Tc
183Ru
178Rh
173Pd
169Ag
165Cd
161In
156Sn
145Sb
133Te
123I
115Xe
1086 Cs
298Ba
253*
Hf
208Ta
200W
193Re
188Os
185Ir
180Pt
177Au
174Hg
171Tl
156Pb
154Bi
143Po
135At
Rn
1207 Fr
Ra
**
Rf
Db
Sg
Bh
Hs
Mt
Ds
Rg
Cn
Uut
Uuq
Uup
Uuh
Uus
Uuo
Lanthanides *
La
Ce
Pr
247Nd
206Pm
205Sm
238Eu
231Gd
233Tb
225Dy
228Ho
Er
226Tm
222Yb
222Lu
217Actinides **
Ac
Th
Pa
U
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
Lr
See also
- Atomic radii of the elements (data page)
- Chemical bond
- Covalent radius
- Bond length
- Steric hindrance
References
- ^ Cotton, F. A.; Wilkinson, G. (1988). Advanced Inorganic Chemistry (5th ed.). Wiley. p. 1385. ISBN 978-0-471-84997-1.
- ^ Basdevant, J.-L.; Rich, J.; Spiro, M. (2005). Fundamentals in Nuclear Physics. Springer. p. 13, fig 1.1. ISBN 978-0-387-01672-6. http://books.google.com/?id=OFx7P9mgC9oC&pg=PA375&dq=helium+%22nuclear+structure%22#PPA13,M1.
- ^ Bragg, W. L. (1920). "The arrangement of atoms in crystals". Philosophical Magazine. 6 40 (236): 169–189.
- ^ Wyckoff, R. W. G. (1923). "On the Hypothesis of Constant Atomic Radii". Proceedings of the National Academy of Sciences of the United States of America 9 (2): 33–38. Bibcode 1923PNAS....9...33W. doi:10.1073/pnas.9.2.33. PMC 1085234. PMID 16576657. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1085234.
- ^ a b c Pauling, L. (1945). The Nature of the Chemical Bond (2nd ed.). Cornell University Press. LCCN 42034474.
- ^ Bohr, N. (1913). "On the Constitution of Atoms and Molecules, Part I. – Binding of Electrons by Positive Nuclei". Philosophical Magazine. 6 26 (151): 1–24. http://web.ihep.su/dbserv/compas/src/bohr13/eng.pdf. Retrieved 8 June 2011.
- ^ Bohr, N. (1913). "On the Constitution of Atoms and Molecules, Part II. – Systems containing only a Single Nucleus". Philosophical Magazine. 6 26 (153): 476–502. http://web.ihep.su/dbserv/compas/src/bohr13b/eng.pdf. Retrieved 8 June 2011.
- ^ Slater, J. C. (1964). "Atomic Radii in Crystals". Journal of Chemical Physics 41 (10): 3199–3205. Bibcode 1964JChPh..41.3199S. doi:10.1063/1.1725697.
- ^ a b Jolly, W. L. (1991). Modern Inorganic Chemistry (2nd ed.). McGraw-Hill. p. 22. ISBN 978-0-0711-2651-9.
- ^ Clementi, E.; Raimond, D. L.; Reinhardt, W. P. (1967). "Atomic Screening Constants from SCF Functions. II. Atoms with 37 to 86 Electrons". Journal of Chemical Physics 47 (4): 1300–1307. Bibcode 1967JChPh..47.1300C. doi:10.1063/1.1712084.
Categories:- Atoms
- Properties of chemical elements
Wikimedia Foundation. 2010.

