- Spinel
-
Spinel 
General Category Oxide minerals - Spinel group Chemical formula MgAl2O4 Strunz classification 04.BB.05 Identification Color Various, red to blue to mauve. Dark green, brown. Black Crystal habit Cubic, octahedral Crystal system Isometric Cleavage Indistinct Fracture Conchoidal, uneven Mohs scale hardness 7.5 – 8.0 Luster Vitreous Streak White Diaphaneity Transparent to translucent Specific gravity 3.6 – 4.1 Optical properties Isotropic Refractive index 1.719 Pleochroism Absent Solubility none Other characteristics Nonmagnetic, non-radioactive, sometimes fluorescent (red) References [1][2] Spinel is the magnesium aluminium member of the larger spinel group of minerals. It has the formula MgAl2O4.[1] Balas ruby is an old name for a rose-tinted variety.
Contents
Spinel group
The spinels are any of a class of minerals of general formulation A2+B23+O42- which crystallise in the cubic (isometric) crystal system, with the oxide anions arranged in a cubic close-packed lattice and the cations A and B occupying some or all of the octahedral and tetrahedral sites in the lattice. A and B can be divalent, trivalent, or quadrivalent cations, including magnesium, zinc, iron, manganese, aluminium, chromium, titanium, and silicon. Although the anion is normally oxide, the analogous thiospinel structure includes the rest of the chalcogenides. A and B can also be the same metal under different charges, such as the case in Fe3O4 (as Fe2+Fe23+O42-).
Members of the spinel group include:[3]
- Aluminium spinels:
- Spinel: MgAl2O4, after which this class of minerals is named
- Gahnite: ZnAl2O4
- Hercynite: FeAl2O4
- Iron spinels:
- Cuprospinel: CuFe2O4
- Franklinite: (Fe,Mn,Zn)(Fe,Mn)2O4
- Jacobsite: MnFe2O4
- Magnetite: Fe3O4
- Trevorite: NiFe2O4
- Ulvöspinel: TiFe2O4
- Zinc ferrite: (Zn, Fe) Fe2O4
- Chromium spinels:
- Chromite: FeCr2O4
- Magnesiochromite: MgCr2O4
- Others with the spinel structure:
- Ringwoodite: (Mg,Fe)2SiO4, an abundant olivine polymorph within the Earth's mantle from about 520 to 660 km depth, and a rare mineral in meteorites
There are many more compounds with a spinel structure, e.g. the thiospinels and selenospinels, that can either be synthesized in the lab or in some cases occur as minerals.
Properties of true spinel
Spinel crystallizes in the isometric system; common crystal forms are octahedra, usually twinned. It has an imperfect octahedral cleavage and a conchoidal fracture. Its hardness is 8, its specific gravity is 3.5-4.1 and it is transparent to opaque with a vitreous to dull luster. It may be colorless, but is usually various shades of red, blue, green, yellow, brown or black. There is a unique natural white spinel, now lost, that surfaced briefly in what is now Sri Lanka. Some spinels are among the most famous gemstones: Among them is the Black Prince's Ruby and the 'Timur ruby' in the British Crown Jewels, and the 'cote de Bretagne' formerly from the French Crown jewels. The Samarian Spinel is the largest known spinel in the world, weighing 500 carats (100 g).
The transparent red spinels were called spinel-rubies or balas rubies. In the past, before the arrival of modern science, spinels and rubies were equally known as rubies. After the 18th century the word ruby was only used for the red gem variety of the mineral corundum and the word spinel became used. "Balas" is derived from Balascia, the ancient name for Badakhshan, a region in central Asia situated in the upper valley of the Kokcha River, one of the principal tributaries of the Oxus River. The Badakshan Province was for centuries the main source for red and pink spinels.
Occurrence
True spinel has long been found in the gemstone-bearing gravel of Sri Lanka and in limestones of the Badakshan Province in modern day Afghanistan and of Mogok in Burma. Recently gem quality spinels were also found in the marbles of Luc Yen (Vietnam), Mahenge and Matombo (Tanzania), Tsavo (Kenya) and in the gravels of Tunduru (Tanzania) and Ilakaka (Madagascar). Spinel is found as a metamorphic mineral, and also as a primary mineral in rare mafic igneous rocks; in these igneous rocks, the magmas are relatively deficient in alkalis relative to aluminium, and aluminium oxide may form as the mineral corundum or may combine with magnesia to form spinel. This is why spinel and ruby are often found together.
Spinel, (Mg,Fe)(Al,Cr)2O4, is common in peridotite in the uppermost Earth's mantle, between approximately 20 km to approximately 120 km kilometers, possibly to lower depths depending on the chromium content[4]. At depths significantly shallower than the Moho, calcic plagioclase is the more stable aluminous mineral in peridotite, while garnet is the stable phase deeper in the mantle.
Spinel, (Mg,Fe)Al2O4, is a common mineral in the Ca-Al-rich inclusions (CAIs) in some chondritic meteorites.
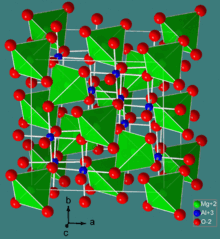
The spinel structure
Normal spinel structures are usually cubic closed-packed oxides with one octahedral and two tetrahedral sites per oxide. The tetrahedral points are smaller than the octahedral points. B3+ ions occupy the octahedral holes because of a charge factor, but can only occupy half of the octahedral holes. A2+ ions occupy 1/8 of the tetrahedral holes. This maximises the lattice energy if the ions are similar in size. A common example of a normal spinel is MgAl2O4.
Inverse spinel structures however are slightly different in that one must take into account the crystal field stabilization energies (CFSE) of the transition metals present. Some ions may have a distinct preference on the octahedral site which is dependent on the d-electron count. If the A2+ ions have a strong preference for the octahedral site, they will force their way into it and displace half of the B3+ ions from the octahedral sites to the tetrahedral sites. If the B3+ ions have a low or zero octahedral site stabilization energy (OSSE), then they have no preference and will adopt the tetrahedral site. A common example of an inverse spinel is Fe3O4, if the Fe2+ (A2+) ions are d6 high-spin and the Fe3+ (B3+) ions are d5 high-spin.
For many years, crystal field theory was invoked to explain the distribution of the cations within the spinels. As the octahedral and tetrahedral sites in the lattice generate different amounts of CFSE, it was argued that the arrangement of the two types of cation that generated the most CFSE would be the most stable. However, this idea was challenged by Burdett and co-workers, who showed that a better treatment used the relative sizes of the s and p atomic orbitals of the two types of atom to determine their site preference.[5] This is because the dominant stabilizing interaction in the solids is not the crystal field stabilization energy generated by the interaction of the ligands with the d-electrons, but the σ-type interactions between the metal cations and the oxide anions. This rationale can explain anomalies in the spinel structures that crystal-field theory cannot, such as the marked preference of Al3+ cations for octahedral sites or of Zn2+ for tetrahedral sites - using crystal field theory would predict that both have no site preference. Only in cases where this size-based approach indicates no preference for one structure over another do crystal field effects make any difference — in effect they are just a small perturbation that can sometimes make a difference, but which often do not.
Synthetic spinel
Synthetic spinel was accidentally produced in the middle of the 18th century, and has been more recently described in scientific publications in 2000 and 2004.[6]
See also
- Ceylonite
- The Samarian Spinel: the largest known spinel in the world, part of the Iranian Crown Jewels
- Black Prince's Ruby
References
- ^ a b Spinel at Mindat
- ^ Spinel at webminerals
- ^ Spinel group at Mindat
- ^ http://www.eeo.ed.ac.uk/homes/sklemme/publications/Klemme_Lithos_2004.pdf
- ^ J.K. Burdett, G.L. Price and S.L. Price (1982). "Role of the crystal-field theory in determining the structures of spinels". J. Am. Chem. Soc. 104: 92–95. doi:10.1021/ja00365a019.
- ^ SSEF: Swiss Gemological Organization. Click Newsletter, Click Flux Grown Synthetic Spinels
Bibliography
- Deer, Howie and Zussman (1966) An Introduction to the Rock-Forming Minerals, Longman, pp. 424–433, ISBN 0-582-44210-9
- Shumann, Walter (2006) Gemstones of the World 3rd edition, Sterling, pp. 116–117.
External links
Categories:- Magnesium minerals
- Aluminium minerals
- Spinel group
- Gemstones
- Cubic minerals
- Aluminium spinels:
Wikimedia Foundation. 2010.